Vascular remodeling of the mouse yolk sac requires hemodynamic force
- PMID: 17720695
- PMCID: PMC4260474
- DOI: 10.1242/dev.02883
Vascular remodeling of the mouse yolk sac requires hemodynamic force
Abstract
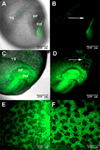
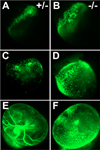
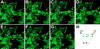
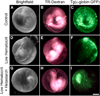
The embryonic heart and vessels are dynamic and form and remodel while functional. Much has been learned about the genetic mechanisms underlying the development of the cardiovascular system, but we are just beginning to understand how changes in heart and vessel structure are influenced by hemodynamic forces such as shear stress. Recent work has shown that vessel remodeling in the mouse yolk sac is secondarily effected when cardiac function is reduced or absent. These findings indicate that proper circulation is required for vessel remodeling, but have not defined whether the role of circulation is to provide mechanical cues, to deliver oxygen or to circulate signaling molecules. Here, we used time-lapse confocal microscopy to determine the role of fluid-derived forces in vessel remodeling in the developing murine yolk sac. Novel methods were used to characterize flows in normal embryos and in embryos with impaired contractility (Mlc2a(-/-)). We found abnormal plasma and erythroblast circulation in these embryos, which led us to hypothesize that the entry of erythroblasts into circulation is a key event in triggering vessel remodeling. We tested this by sequestering erythroblasts in the blood islands, thereby lowering the hematocrit and reducing shear stress, and found that vessel remodeling and the expression of eNOS (Nos3) depends on erythroblast flow. Further, we rescued remodeling defects and eNOS expression in low-hematocrit embryos by restoring the viscosity of the blood. These data show that hemodynamic force is necessary and sufficient to induce vessel remodeling in the mammalian yolk sac.
Figures





References
-
- Akers W, Haidekker MA. A molecular rotor as viscosity sensor in aqueous colloid solutions. J. Biomech. Eng. 2004;126:340–345. - PubMed
-
- Argraves WS, Drake CJ. Genes critical to vasculogenesis as defined by systematic analysis of vascular defects in knockout mice. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005;286:875–884. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. - PubMed
-
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. - PubMed
-
- Cheng C, van Haperen R, de Waard M, van Damme LC, Tempel D, Hanemaaijer L, van Cappellen GW, Bos J, Slager CJ, Duncker DJ, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

