Regulation of one-carbon metabolism in Arabidopsis: the N-terminal regulatory domain of cystathionine gamma-synthase is cleaved in response to folate starvation
- PMID: 17720756
- PMCID: PMC2048731
- DOI: 10.1104/pp.107.105379
Regulation of one-carbon metabolism in Arabidopsis: the N-terminal regulatory domain of cystathionine gamma-synthase is cleaved in response to folate starvation
Abstract
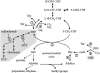
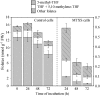
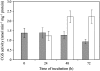
In all organisms, control of folate homeostasis is of vital importance to sustain the demand for one-carbon (C1) units that are essential in major metabolic pathways. In this study we induced folate deficiency in Arabidopsis (Arabidopsis thaliana) cells by using two antifolate inhibitors. This treatment triggered a rapid and important decrease in the pool of folates with significant modification in the distribution of C1-substituted folate coenzymes, suggesting an adaptive response to favor a preferential shuttling of the flux of C1 units to the synthesis of nucleotides over the synthesis of methionine (Met). Metabolic profiling of folate-deficient cells indicated important perturbation of the activated methyl cycle because of the impairment of Met synthases that are deprived of their substrate 5-methyl-tetrahydrofolate. Intriguingly, S-adenosyl-Met and Met pools declined during the initial period of folate starvation but were further restored to typical levels. Reestablishment of Met and S-adenosyl-Met homeostasis was concomitant with a previously unknown posttranslational modification that consists in the removal of 92 amino acids at the N terminus of cystathionine gamma-synthase (CGS), the first specific enzyme for Met synthesis. Rescue experiments and analysis of different stresses indicated that CGS processing is specifically associated with perturbation of the folates pool. Also, CGS processing involves chloroplastic serine-type proteases that are expressed in various plant species subjected to folate starvation. We suggest that a metabolic effector, to date unidentified, can modulate CGS activity in vivo through an interaction with the N-terminal domain of the enzyme and that removal of this domain can suppress this regulation.
Figures





Similar articles
-
A genome-wide and metabolic analysis determined the adaptive response of Arabidopsis cells to folate depletion induced by methotrexate.Plant Physiol. 2008 Dec;148(4):2083-95. doi: 10.1104/pp.108.130336. Epub 2008 Oct 17. Plant Physiol. 2008. PMID: 18931140 Free PMC article.
-
Constitutive overexpression of cystathionine gamma-synthase in Arabidopsis leads to accumulation of soluble methionine and S-methylmethionine.Plant Physiol. 2002 Jan;128(1):95-107. Plant Physiol. 2002. PMID: 11788756 Free PMC article.
-
Differential response of methionine metabolism in two grain legumes, soybean and azuki bean, expressing a mutated form of Arabidopsis cystathionine γ-synthase.J Plant Physiol. 2013 Feb 15;170(3):338-45. doi: 10.1016/j.jplph.2012.10.018. Epub 2012 Dec 31. J Plant Physiol. 2013. PMID: 23286999
-
Folic acid metabolism and its disruption by pharmacologic agents.NCI Monogr. 1987;(5):1-8. NCI Monogr. 1987. PMID: 3124003 Review.
-
Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis.Biochem J. 2013 Apr 15;451(2):145-54. doi: 10.1042/BJ20121744. Biochem J. 2013. PMID: 23535167 Review.
Cited by
-
The plastidial folylpolyglutamate synthetase and root apical meristem maintenance.Plant Signal Behav. 2011 May;6(5):751-4. doi: 10.4161/psb.6.5.15403. Epub 2011 May 1. Plant Signal Behav. 2011. PMID: 21502816 Free PMC article.
-
The folylpolyglutamate synthetase plastidial isoform is required for postembryonic root development in Arabidopsis.Plant Physiol. 2011 Mar;155(3):1237-51. doi: 10.1104/pp.110.168278. Epub 2011 Jan 13. Plant Physiol. 2011. PMID: 21233333 Free PMC article.
-
C1 Metabolism Inhibition and Nitrogen Deprivation Trigger Triacylglycerol Accumulation in Arabidopsis thaliana Cell Cultures and Highlight a Role of NPC in Phosphatidylcholine-to-Triacylglycerol Pathway.Front Plant Sci. 2017 Jan 4;7:2014. doi: 10.3389/fpls.2016.02014. eCollection 2016. Front Plant Sci. 2017. PMID: 28101097 Free PMC article.
-
Metabolic model of central carbon and energy metabolisms of growing Arabidopsis thaliana in relation to sucrose translocation.BMC Plant Biol. 2016 Dec 28;16(1):262. doi: 10.1186/s12870-016-0868-3. BMC Plant Biol. 2016. PMID: 28031032 Free PMC article.
-
Deep transcriptome sequencing of rhizome and aerial-shoot in Sorghum propinquum.Plant Mol Biol. 2014 Feb;84(3):315-27. doi: 10.1007/s11103-013-0135-z. Epub 2013 Oct 9. Plant Mol Biol. 2014. PMID: 24104862
References
-
- Adam Z, Rudella A, van Wijk KJ (2006) Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr Opin Plant Biol 9 234–240 - PubMed
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 - PubMed
-
- Castro R, Struys EA, Jansen EEW, Blom HJ, de Almeida IT, Jakobs C (2002) Quantification of plasma S-adenosylmethionine and S-adenosylhomocysteine as their fluorescent 1,N-6-etheno derivatives: an adaptation of previously described methodology. J Pharm Biomed Anal 29 963–968 - PubMed
-
- Chiba Y, Ishikawa M, Kijima F, Tyson RH, Kim J, Yamamoto A, Nambara E, Leustek T, Wallsgrove RM, Naito S (1999) Evidence for autoregulation of cystathionine gamma-synthase mRNA stability in Arabidopsis. Science 286 1371–1374 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

