gerT, a newly discovered germination gene under the control of the sporulation transcription factor sigmaK in Bacillus subtilis
- PMID: 17720779
- PMCID: PMC2168713
- DOI: 10.1128/JB.01053-07
gerT, a newly discovered germination gene under the control of the sporulation transcription factor sigmaK in Bacillus subtilis
Abstract
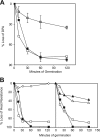
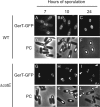
We report the identification of a gene, herein designated gerT (formerly yozR), that is involved in germination by spores of Bacillus subtilis. The gerT gene is induced late in sporulation under the positive control of the transcription factor sigma(K) and under the negative control of the DNA-binding protein GerE. The gerT gene product (GerT) is a component of the spore coat, and its incorporation into the coat takes place in two stages. GerT initially assembles into foci, which then spread around the developing spore in a process that is dependent on the morphogenetic protein CotE. Mutant spores lacking GerT respond poorly to multiple germinants and are impaired at an early stage of germination.
Figures



Similar articles
-
Maintaining the transcription factor SpoIIID level late during sporulation causes spore defects in Bacillus subtilis.J Bacteriol. 2007 Oct;189(20):7302-9. doi: 10.1128/JB.00839-07. Epub 2007 Aug 10. J Bacteriol. 2007. PMID: 17693499 Free PMC article.
-
A spore coat protein, CotS, of Bacillus subtilis is synthesized under the regulation of sigmaK and GerE during development and is located in the inner coat layer of spores.J Bacteriol. 1998 Jun;180(11):2968-74. doi: 10.1128/JB.180.11.2968-2974.1998. J Bacteriol. 1998. PMID: 9603889 Free PMC article.
-
Sporulation-specific expression of the yvgW (cadA) gene and the effect of blockage on spore properties in Bacillus subtilis.Gene. 2006 Nov 1;382:71-8. doi: 10.1016/j.gene.2006.06.014. Epub 2006 Jul 5. Gene. 2006. PMID: 16901659
-
[Overview of study on Bacillus subtilis spores].Yakugaku Zasshi. 2013;133(7):783-97. doi: 10.1248/yakushi.13-00143. Yakugaku Zasshi. 2013. PMID: 23811766 Review. Japanese.
-
Recent progress in proteins regulating the germination of Bacillus subtilis spores.J Bacteriol. 2025 Feb 20;207(2):e0028524. doi: 10.1128/jb.00285-24. Epub 2025 Jan 8. J Bacteriol. 2025. PMID: 39772627 Free PMC article. Review.
Cited by
-
Germinant generation from δ-endotoxin of Bacillus thuringiensis strain 1.1.Curr Microbiol. 2011 May;62(5):1431-7. doi: 10.1007/s00284-011-9878-4. Epub 2011 Feb 1. Curr Microbiol. 2011. PMID: 21286721
-
Small proteins link coat and cortex assembly during sporulation in Bacillus subtilis.Mol Microbiol. 2012 May;84(4):682-96. doi: 10.1111/j.1365-2958.2012.08052.x. Epub 2012 Apr 18. Mol Microbiol. 2012. PMID: 22463703 Free PMC article.
-
Molecular Physiological Characterization of a High Heat Resistant Spore Forming Bacillus subtilis Food Isolate.Microorganisms. 2021 Mar 23;9(3):667. doi: 10.3390/microorganisms9030667. Microorganisms. 2021. PMID: 33807113 Free PMC article.
-
Replication-Transcription Conflicts Generate R-Loops that Orchestrate Bacterial Stress Survival and Pathogenesis.Cell. 2017 Aug 10;170(4):787-799.e18. doi: 10.1016/j.cell.2017.07.044. Cell. 2017. PMID: 28802046 Free PMC article.
-
The Bacillus cereus GerN and GerT protein homologs have distinct roles in spore germination and outgrowth, respectively.J Bacteriol. 2008 Sep;190(18):6148-52. doi: 10.1128/JB.00789-08. Epub 2008 Jul 18. J Bacteriol. 2008. PMID: 18641133 Free PMC article.
References
-
- Driks, A. 2002. Proteins of the spore core and coat, p. 527-536. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM PRESS, Washington, DC.
-
- Driks, A., S. Roels, B. Beall, C. P. Moran, Jr., and R. Losick. 1994. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 8:234-244. - PubMed
-
- Driks, A., and P. Setlow. 2000. Morphogenesis and properties of the bacterial spore, p. 191-218. In Y. V. Brun and L. J. Shimkets, (ed.), Prokaryotic development. American Society for Microbiology, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

