A TolC-like protein is required for heterocyst development in Anabaena sp. strain PCC 7120
- PMID: 17720784
- PMCID: PMC2168721
- DOI: 10.1128/JB.00750-07
A TolC-like protein is required for heterocyst development in Anabaena sp. strain PCC 7120
Abstract
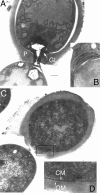
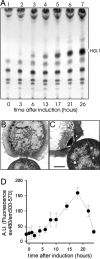
The filamentous cyanobacterium Anabaena sp. strain PCC 7120 forms heterocysts in a semiregular pattern when it is grown on N2 as the sole nitrogen source. The transition from vegetative cells to heterocysts requires marked metabolic and morphological changes. We show that a trimeric pore-forming outer membrane beta-barrel protein belonging to the TolC family, Alr2887, is up-regulated in developing heterocysts and is essential for diazotrophic growth. Mutants defective in Alr2887 did not form the specific glycolipid layer of the heterocyst cell wall, which is necessary to protect nitrogenase from external oxygen. Comparison of the glycolipid contents of wild-type and mutant cells indicated that the protein is not involved in the synthesis of glycolipids but might instead serve as an exporter for the glycolipid moieties or enzymes involved in glycolipid attachment. We propose that Alr2887, together with an ABC transporter like DevBCA, is part of a protein export system essential for assembly of the heterocyst glycolipid layer. We designate the alr2887 gene hgdD (heterocyst glycolipid deposition protein).
Figures




Similar articles
-
PrpJ, a PP2C-type protein phosphatase located on the plasma membrane, is involved in heterocyst maturation in the cyanobacterium Anabaena sp. PCC 7120.Mol Microbiol. 2007 Apr;64(2):347-58. doi: 10.1111/j.1365-2958.2007.05654.x. Epub 2007 Mar 19. Mol Microbiol. 2007. PMID: 17371502
-
All0809/8/7 is a DevBCA-like ABC-type efflux pump required for diazotrophic growth in Anabaena sp. PCC 7120.Microbiology (Reading). 2012 Oct;158(Pt 10):2537-2545. doi: 10.1099/mic.0.058909-0. Epub 2012 Aug 2. Microbiology (Reading). 2012. PMID: 22859614
-
Novel ATP-driven pathway of glycolipid export involving TolC protein.J Biol Chem. 2011 Nov 4;286(44):38202-38210. doi: 10.1074/jbc.M111.269332. Epub 2011 Sep 14. J Biol Chem. 2011. PMID: 21917923 Free PMC article.
-
Roles of DevBCA-like ABC transporters in the physiology of Anabaena sp. PCC 7120.Int J Med Microbiol. 2019 Jul;309(5):325-330. doi: 10.1016/j.ijmm.2019.04.005. Epub 2019 Apr 28. Int J Med Microbiol. 2019. PMID: 31133373 Review.
-
The cell wall in heterocyst formation by Anabaena sp. PCC 7120.J Basic Microbiol. 2009 Feb;49(1):5-24. doi: 10.1002/jobm.200800300. J Basic Microbiol. 2009. PMID: 19253332 Review.
Cited by
-
Structure, Assembly, and Function of Tripartite Efflux and Type 1 Secretion Systems in Gram-Negative Bacteria.Chem Rev. 2021 May 12;121(9):5479-5596. doi: 10.1021/acs.chemrev.1c00055. Epub 2021 Apr 28. Chem Rev. 2021. PMID: 33909410 Free PMC article. Review.
-
Compartmentalized function through cell differentiation in filamentous cyanobacteria.Nat Rev Microbiol. 2010 Jan;8(1):39-50. doi: 10.1038/nrmicro2242. Nat Rev Microbiol. 2010. PMID: 19966815 Review.
-
Extracellular Vesicles: An Overlooked Secretion System in Cyanobacteria.Life (Basel). 2020 Jul 31;10(8):129. doi: 10.3390/life10080129. Life (Basel). 2020. PMID: 32751844 Free PMC article. Review.
-
Identification of protein secretion systems in bacterial genomes.Sci Rep. 2016 Mar 16;6:23080. doi: 10.1038/srep23080. Sci Rep. 2016. PMID: 26979785 Free PMC article.
-
Structure and conservation of the periplasmic targeting factor Tic22 protein from plants and cyanobacteria.J Biol Chem. 2012 Jul 13;287(29):24164-73. doi: 10.1074/jbc.M112.341644. Epub 2012 May 16. J Biol Chem. 2012. PMID: 22593581 Free PMC article.
References
-
- Adams, D. G., and P. S. Duggan. 1999. Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 144:3-33.
-
- Bauer, C. C., K. S. Ramaswamy, S. Endley, L. A. Scappino, J. W. Golden, and R. Haselkorn. 1997. Suppression of heterocyst differentiation in Anabaena PCC 7120 by a cosmid carrying wild-type genes encoding enzymes for fatty acid synthesis. FEMS Microbiol. Lett. 151:23-30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

