Membrane-bound, 2-keto-D-gluconate-yielding D-gluconate dehydrogenase from "Gluconobacter dioxyacetonicus" IFO 3271: molecular properties and gene disruption
- PMID: 17720837
- PMCID: PMC2075040
- DOI: 10.1128/AEM.00493-07
Membrane-bound, 2-keto-D-gluconate-yielding D-gluconate dehydrogenase from "Gluconobacter dioxyacetonicus" IFO 3271: molecular properties and gene disruption
Abstract
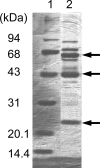
Most Gluconobacter species produce and accumulate 2-keto-d-gluconate (2KGA) and 5KGA simultaneously from d-glucose via GA in culture medium. 2KGA is produced by membrane-bound flavin adenine dinucleotide-containing GA 2-dehydrogenase (FAD-GADH). FAD-GADH was purified from "Gluconobacter dioxyacetonicus" IFO 3271, and N-terminal sequences of the three subunits were analyzed. PCR primers were designed from the N-terminal sequences, and part of the FAD-GADH genes was cloned as a PCR product. Using this PCR product, gene fragments containing whole FAD-GADH genes were obtained, and finally the nucleotide sequence of 9,696 bp was determined. The cloned sequence had three open reading frames (ORFs), gndS, gndL, and gndC, corresponding to small, large, and cytochrome c subunits of FAD-GADH, respectively. Seven other ORFs were also found, one of which showed identity to glucono-delta-lactonase, which might be involved directly in 2KGA production. Three mutant strains defective in either gndL or sldA (the gene responsible for 5KGA production) or both were constructed. Ferricyanide-reductase activity with GA in the membrane fraction of the gndL-defective strain decreased by about 60% of that of the wild-type strain, while in the sldA-defective strain, activity with GA did not decrease and activities with glycerol, d-arabitol, and d-sorbitol disappeared. Unexpectedly, the strain defective in both gndL and sldA (double mutant) still showed activity with GA. Moreover, 2KGA production was still observed in gndL and double mutant strains. 5KGA production was not observed at all in sldA and double mutant strains. Thus, it seems that "G. dioxyacetonicus" IFO 3271 has another membrane-bound enzyme that reacts with GA, producing 2KGA.
Figures


Similar articles
-
Screening of thermotolerant Gluconobacter strains for production of 5-keto-D-gluconic acid and disruption of flavin adenine dinucleotide-containing D-gluconate dehydrogenase.Appl Environ Microbiol. 2009 Jul;75(13):4240-7. doi: 10.1128/AEM.00640-09. Epub 2009 May 1. Appl Environ Microbiol. 2009. PMID: 19411430 Free PMC article.
-
Preparation of enzymes required for enzymatic quantification of 5-keto-D-gluconate and 2-keto-D-gluconate.Biosci Biotechnol Biochem. 2007 Oct;71(10):2478-86. doi: 10.1271/bbb.70259. Epub 2007 Oct 7. Biosci Biotechnol Biochem. 2007. PMID: 17928715
-
Efficient Production of 2,5-Diketo-d-Gluconate via Heterologous Expression of 2-Ketogluconate Dehydrogenase in Gluconobacter japonicus.Appl Environ Microbiol. 2015 May 15;81(10):3552-60. doi: 10.1128/AEM.04176-14. Epub 2015 Mar 13. Appl Environ Microbiol. 2015. PMID: 25769838 Free PMC article.
-
New developments in oxidative fermentation.Appl Microbiol Biotechnol. 2003 Feb;60(6):643-53. doi: 10.1007/s00253-002-1155-9. Epub 2002 Dec 18. Appl Microbiol Biotechnol. 2003. PMID: 12664142 Review.
-
Ketogluconate production by Gluconobacter strains: enzymes and biotechnological applications.Biosci Biotechnol Biochem. 2024 Apr 22;88(5):499-508. doi: 10.1093/bbb/zbae013. Biosci Biotechnol Biochem. 2024. PMID: 38323387 Review.
Cited by
-
Characteristics of Metabolites in the Development of Atherosclerosis in Tibetan Minipigs Determined Using Untargeted Metabolomics.Nutrients. 2023 Oct 18;15(20):4425. doi: 10.3390/nu15204425. Nutrients. 2023. PMID: 37892500 Free PMC article.
-
2-ketogluconic acid secretion by incorporation of Pseudomonas putida KT 2440 gluconate dehydrogenase (gad) operon in Enterobacter asburiae PSI3 improves mineral phosphate solubilization.Curr Microbiol. 2013 Sep;67(3):388-94. doi: 10.1007/s00284-013-0372-z. Epub 2013 May 11. Curr Microbiol. 2013. PMID: 23666029
-
Oxidative Fermentation of Acetic Acid Bacteria and Its Products.Front Microbiol. 2022 May 24;13:879246. doi: 10.3389/fmicb.2022.879246. eCollection 2022. Front Microbiol. 2022. PMID: 35685922 Free PMC article. Review.
-
Compartmentalized glucose metabolism in Pseudomonas putida is controlled by the PtxS repressor.J Bacteriol. 2010 Sep;192(17):4357-66. doi: 10.1128/JB.00520-10. Epub 2010 Jun 25. J Bacteriol. 2010. PMID: 20581202 Free PMC article.
-
Efficient biosynthesis of 2-keto-D-gluconic acid by fed-batch culture of metabolically engineered Gluconobacter japonicus.Synth Syst Biotechnol. 2019 Jul 27;4(3):134-141. doi: 10.1016/j.synbio.2019.07.001. eCollection 2019 Sep. Synth Syst Biotechnol. 2019. PMID: 31384676 Free PMC article.
References
-
- Ameyama, M., O. Adachi, and A. W. Willis. 1982. [33] 5-Keto-d-gluconate reductase from Gluconobacter suboxydans. Methods Enzymol. 89:198-202.
-
- Ameyama, M., O. Adachi, and A. W. Willis. 1982. [34] 2-Keto-d-gluconate reductase from acetic acid bacteria. Methods Enzymol. 89:203-210. - PubMed
-
- Berks, B. C., F. Sargent, and T. Palmer. 2000. The Tat protein export pathway. Mol. Microbiol. 35:260-274. - PubMed
-
- Dulley, J. R., and P. A. Grieve. 1975. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal. Biochem. 64:136-141. - PubMed
-
- Elfari, M., S. W. Ha, C. Bremus, M. Merfort, V. Khodaverdi, U. Herrmann, H. Sahm, and H. Gorisch. 2005. A Gluconobacter oxydans mutant converting glucose almost quantitatively to 5-keto-d-gluconic acid. Appl. Microbiol. Biotechnol. 66:668-674. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

