Immunoglobulins to surface-associated biofilm immunogens provide a novel means of visualization of methicillin-resistant Staphylococcus aureus biofilms
- PMID: 17720840
- PMCID: PMC2075055
- DOI: 10.1128/AEM.00855-07
Immunoglobulins to surface-associated biofilm immunogens provide a novel means of visualization of methicillin-resistant Staphylococcus aureus biofilms
Abstract
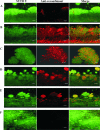
Antigens from the methicillin-resistant Staphylococcus aureus (MRSA) cell wall have been shown to be immunogenic in vivo and upregulated during biofilm growth. In this study, we created purified, recombinant forms of selected antigens and biofilm-upregulated, cell wall-associated proteins. These proteins were shown to cause a robust polyclonal immunoglobulin G (IgG) response when used to immunize rabbits. Antibodies against these recombinant proteins bound to the native forms of each protein as harvested from in vitro grown biofilms of MRSA, as determined both via Western blot analysis and immunofluorescence confocal microscopy. These IgGs could be utilized as imaging tools that localize to areas of specific protein production within a biofilm. This work illustrates that immunogenic, cell wall-associated, biofilm-upregulated proteins are promising for in vitro visualization of biofilm growth, architecture, and space-function relationships.
Figures
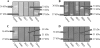

References
-
- Biswas, R., L. Voggu, U. K. Simon, P. Hentschel, G. Thumm, and F. Gotz. 2006. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 259:260-268. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

