Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis
- PMID: 17720869
- PMCID: PMC2002617
- DOI: 10.1105/tpc.107.050716
Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis
Abstract
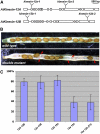
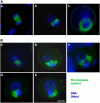
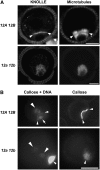
In plant cells, cytokinesis is brought about by the phragmoplast. The phragmoplast has a dynamic microtubule array of two mirrored sets of microtubules, which are aligned perpendicularly to the division plane with their plus ends located at the division site. It is not well understood how the phragmoplast microtubule array is organized. In Arabidopsis thaliana, two homologous microtubule motor kinesins, PAKRP1/Kinesin-12A and PAKRP1L/Kinesin-12B, localize exclusively at the juxtaposing plus ends of the antiparallel microtubules in the middle region of the phragmoplast. When either kinesin was knocked out by T-DNA insertions, mutant plants did not show a noticeable defect. However, in the absence of both kinesins, postmeiotic development of the male gametophyte was severely inhibited. In dividing microspores of the double mutant, microtubules often became disorganized following chromatid segregation and failed to form an antiparallel microtubule array between reforming nuclei. Consequently, the first postmeiotic cytokinesis was abolished without the formation of a cell plate, which led to failures in the birth of the generative cell and, subsequently, the sperm. Thus, our results indicate that Kinesin-12A and Kinesin-12B jointly play a critical role in the organization of phragmoplast microtubules during cytokinesis in the microspore that is essential for cell plate formation. Furthermore, we conclude that Kinesin-12 members serve as dynamic linkers of the plus ends of antiparallel microtubules in the phragmoplast.
Figures



Similar articles
-
Arabidopsis Fused kinase and the Kinesin-12 subfamily constitute a signalling module required for phragmoplast expansion.Plant J. 2012 Oct;72(2):308-19. doi: 10.1111/j.1365-313X.2012.05077.x. Epub 2012 Jul 24. Plant J. 2012. PMID: 22709276
-
Interaction of antiparallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis.Plant Cell. 2011 Aug;23(8):2909-23. doi: 10.1105/tpc.110.078204. Epub 2011 Aug 26. Plant Cell. 2011. PMID: 21873565 Free PMC article.
-
Identification of a phragmoplast-associated kinesin-related protein in higher plants.Curr Biol. 2000 Jun 29;10(13):797-800. doi: 10.1016/s0960-9822(00)00564-9. Curr Biol. 2000. PMID: 10898978
-
Plant Kinesin-12: Localization Heterogeneity and Functional Implications.Int J Mol Sci. 2019 Aug 28;20(17):4213. doi: 10.3390/ijms20174213. Int J Mol Sci. 2019. PMID: 31466291 Free PMC article. Review.
-
Kinesin motors in plants: from subcellular dynamics to motility regulation.Curr Opin Plant Biol. 2015 Dec;28:120-6. doi: 10.1016/j.pbi.2015.10.003. Epub 2015 Nov 8. Curr Opin Plant Biol. 2015. PMID: 26556761 Review.
Cited by
-
Are kinesins required for organelle trafficking in plant cells?Front Plant Sci. 2012 Jul 24;3:170. doi: 10.3389/fpls.2012.00170. eCollection 2012. Front Plant Sci. 2012. PMID: 22837763 Free PMC article.
-
Arabidopsis kinesin KP1 specifically interacts with VDAC3, a mitochondrial protein, and regulates respiration during seed germination at low temperature.Plant Cell. 2011 Mar;23(3):1093-106. doi: 10.1105/tpc.110.082420. Epub 2011 Mar 15. Plant Cell. 2011. PMID: 21406623 Free PMC article.
-
Evaluating the microtubule cytoskeleton and its interacting proteins in monocots by mining the rice genome.Ann Bot. 2009 Feb;103(3):387-402. doi: 10.1093/aob/mcn248. Epub 2008 Dec 23. Ann Bot. 2009. PMID: 19106179 Free PMC article. Review.
-
Nucleoporin MOS7/Nup88 is required for mitosis in gametogenesis and seed development in Arabidopsis.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):18393-8. doi: 10.1073/pnas.1421911112. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489100 Free PMC article.
-
Genome-wide identification and expression analysis of the kinesin gene superfamily suggests roles in response to abiotic stress and fertility of wheat (Triticum aestivum L.).BMC Genomics. 2024 Dec 19;25(1):1223. doi: 10.1186/s12864-024-11156-7. BMC Genomics. 2024. PMID: 39701941 Free PMC article.
References
-
- Asada, T., Kuriyama, R., and Shibaoka, H. (1997). TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J. Cell Sci. 110 179–189. - PubMed
-
- Asada, T., Sonobe, S., and Shibaoka, H. (1991). Microtubule translocation in the cytokinetic apparatus of cultured tobacco cells. Nature 350 238–241.
-
- Austin, J.R., Segui-Simarro, J.M., and Staehelin, L.A. (2005). Quantitative analysis of changes in spatial distribution and plus-end geometry of microtubules involved in plant-cell cytokinesis. J. Cell Sci. 118 3895–3903. - PubMed
-
- Barroso, C., Chan, J., Allan, V., Doonan, J., Hussey, P., and Lloyd, C. (2000). Two kinesin-related proteins associated with the cold-stable cytoskeleton of carrot cells: Characterization of a novel kinesin, DcKRP120–2. Plant J. 24 859–868. - PubMed
-
- Boleti, H., Karsenti, E., and Vernos, I. (1996). Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell 84 49–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

