Terminal regions of wheat chromosomes select their pairing partners in meiosis
- PMID: 17720899
- PMCID: PMC2034636
- DOI: 10.1534/genetics.107.078121
Terminal regions of wheat chromosomes select their pairing partners in meiosis
Abstract
Many plant species, including important crops like wheat, are polyploids that carry more than two sets of genetically related chromosomes capable of meiotic pairing. To safeguard a diploid-like behavior at meiosis, many polyploids evolved genetic loci that suppress incorrect pairing and recombination of homeologues. The Ph1 locus in wheat was proposed to ensure homologous pairing by controlling the specificity of centromere associations that precede chromosome pairing. Using wheat chromosomes that carry rye centromeres, we show that the centromere associations in early meiosis are not based on homology and that the Ph1 locus has no effect on such associations. Although centromeres indeed undergo a switch from nonhomologous to homologous associations in meiosis, this process is driven by the terminally initiated synapsis. The centromere has no effect on metaphase I chiasmate chromosome associations: homologs with identical or different centromeres, in the presence and absence of Ph1, pair the same. A FISH analysis of the behavior of centromeres and distal chromomeres in telocentric and bi-armed chromosomes demonstrates that it is not the centromeric, but rather the subtelomeric, regions that are involved in the correct partner recognition and selection.
Figures
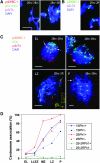
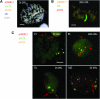
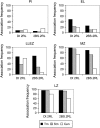
Similar articles
-
The Ph1 locus is needed to ensure specific somatic and meiotic centromere association.Nature. 2001 May 10;411(6834):204-7. doi: 10.1038/35075597. Nature. 2001. PMID: 11346798
-
CENH3 morphogenesis reveals dynamic centromere associations during synaptonemal complex formation and the progression through male meiosis in hexaploid wheat.Plant J. 2017 Jan;89(2):235-249. doi: 10.1111/tpj.13379. Epub 2017 Jan 17. Plant J. 2017. PMID: 27624968
-
Control of conformation changes associated with homologue recognition during meiosis.Theor Appl Genet. 2005 Aug;111(3):505-10. doi: 10.1007/s00122-005-2040-6. Epub 2005 May 14. Theor Appl Genet. 2005. PMID: 15895201
-
Centromere Associations in Meiotic Chromosome Pairing.Annu Rev Genet. 2015;49:95-114. doi: 10.1146/annurev-genet-112414-055107. Epub 2015 Sep 29. Annu Rev Genet. 2015. PMID: 26421510 Review.
-
Homologous pairing and chromosome dynamics in meiosis and mitosis.Biochim Biophys Acta. 2004 Mar 15;1677(1-3):165-80. doi: 10.1016/j.bbaexp.2003.11.017. Biochim Biophys Acta. 2004. PMID: 15020057 Review.
Cited by
-
Detailed recombination studies along chromosome 3B provide new insights on crossover distribution in wheat (Triticum aestivum L.).Genetics. 2009 Feb;181(2):393-403. doi: 10.1534/genetics.108.097469. Epub 2008 Dec 8. Genetics. 2009. PMID: 19064706 Free PMC article.
-
Prelude to a division.Annu Rev Cell Dev Biol. 2008;24:397-424. doi: 10.1146/annurev.cellbio.23.090506.123245. Annu Rev Cell Dev Biol. 2008. PMID: 18597662 Free PMC article. Review.
-
Chromosome-nuclear envelope tethering - a process that orchestrates homologue pairing during plant meiosis?J Cell Sci. 2020 Aug 12;133(15):jcs243667. doi: 10.1242/jcs.243667. J Cell Sci. 2020. PMID: 32788229 Free PMC article. Review.
-
Extensive homoeologous genome exchanges in allopolyploid crops revealed by mRNAseq-based visualization.Plant Biotechnol J. 2017 May;15(5):594-604. doi: 10.1111/pbi.12657. Epub 2016 Dec 6. Plant Biotechnol J. 2017. PMID: 27808473 Free PMC article.
-
Chromosome fusion and programmed DNA elimination shape karyotypes of parasitic nematodes.bioRxiv [Preprint]. 2023 Dec 23:2023.12.21.572835. doi: 10.1101/2023.12.21.572835. bioRxiv. 2023. Update in: Curr Biol. 2024 May 20;34(10):2147-2161.e5. doi: 10.1016/j.cub.2024.04.022. PMID: 38187595 Free PMC article. Updated. Preprint.
References
-
- Albini, S. M., and G. H. Jones, 1987. Synaptonemal complex spreading in Allium cepa and A. Fistulosum. I. The initiation and sequence of pairing. Chromosoma 95: 324–338.
-
- Aragón-Alcaide, L., S. Reader, A. Beven, P. Shaw, T. Miller et al., 1997. Association of homologous chromosomes during floral development. Curr. Biol. 7: 905–908. - PubMed
-
- Bass, H. W., O. Riera-Lizarazu, E. V. Ananiev, S. J. Bordoli, H. W. Rines et al., 2000. Evidence for the coincident initiation of homolog pairing and synapsis during the telomere-clustering (bouquet) stage of meiotic prophase. J. Cell Sci. 113: 1033–1042. - PubMed
-
- Bedbrook, J. R., J. Jones, M. O'Dell, R. Thompson and R. B. Flavell, 1980. A molecular description of telomeric heterochromatin in Secale species. Cell 19: 545–560. - PubMed
-
- Bennett, M. D., J. B. Smith, S. Simpson and B. Wells, 1979. Intranuclear fibrillar material in cereal pollen mother cells. Chromosoma 71: 289–332.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

