Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases
- PMID: 17720922
- PMCID: PMC2034658
- DOI: 10.1534/genetics.107.075846
Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases
Abstract
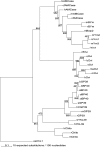
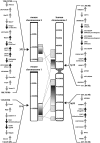
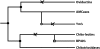
Family 18 of glycosyl hydrolases encompasses chitinases and so-called chi-lectins lacking enzymatic activity due to amino acid substitutions in their active site. Both types of proteins widely occur in mammals although these organisms lack endogenous chitin. Their physiological function(s) as well as evolutionary relationships are still largely enigmatic. An overview of all family members is presented and their relationships are described. Molecular phylogenetic analyses suggest that both active chitinases (chitotriosidase and AMCase) result from an early gene duplication event. Further duplication events, followed by mutations leading to loss of chitinase activity, allowed evolution of the chi-lectins. The homologous genes encoding chitinase(-like) proteins are clustered in two distinct loci that display a high degree of synteny among mammals. Despite the shared chromosomal location and high homology, individual genes have evolved independently. Orthologs are more closely related than paralogues, and calculated substitution rate ratios indicate that protein-coding sequences underwent purifying selection. Substantial gene specialization has occurred in time, allowing for tissue-specific expression of pH optimized chitinases and chi-lectins. Finally, several family 18 chitinase-like proteins are present only in certain lineages of mammals, exemplifying recent evolutionary events in the chitinase protein family.
Figures



References
-
- Badariotti, F., M. Kypriotou, C. Lelong, M. P. Dubos, E. Renard et al., 2006. The phylogenetically conserved molluscan chitinase-like protein 1 (Cg-Clp1), homologue of human HC-gp39, stimulates proliferation and regulates synthesis of extracellular matrix components of mammalian chondrocytes. J. Biol. Chem. 281: 29583–29596. - PubMed
-
- Beutler, E., and G. A. Grabowski, 1995. Gaucher disease, pp. 2641–2670 in The Metabolic and Molecular Bases of Inherited Disease, Ed. 7, edited by C. R. Scriver, A. L. Beaudet, W. S. Sly and D. Valle. McGraw-Hill, New York.
-
- Boot, R. G., G. H. Renkema, A. Strijland, A. J. van Zonneveld and J. M. Aerts, 1995. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J. Biol. Chem. 270: 26252–26256. - PubMed
-
- Boot, R. G., G. H. Renkema, M. Verhoek, A. Strijland, J. Bliek et al., 1998. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J. Biol. Chem. 273: 25680–25685. - PubMed
-
- Boot, R. G., E. F. Blommaart, E. Swart, K. Ghauharali-van der Vlugt, N. Bijl et al., 2001. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 276: 6770–6778. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

