Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction
- PMID: 17720974
- PMCID: PMC2169496
- DOI: 10.1074/jbc.M704085200
Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction
Abstract
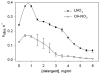
Fatty acid nitration by nitric oxide-derived species yields electrophilic products that adduct protein thiols, inducing changes in protein function and distribution. Nitro-fatty acid adducts of protein and reduced glutathione (GSH) are detected in healthy human blood. Kinetic and mass spectrometric analyses reveal that nitroalkene derivatives of oleic acid (OA-NO2) and linoleic acid (LNO2) rapidly react with GSH and Cys via Michael addition reaction. Rates of OA-NO2 and LNO2 reaction with GSH, determined via stopped flow spectrophotometry, displayed second-order rate constants of 183 M(-1)S(-1) and 355 M(-1)S(-1), respectively, at pH 7.4 and 37 degrees C. These reaction rates are significantly greater than those for GSH reaction with hydrogen peroxide and non-nitrated electrophilic fatty acids including 8-iso-prostaglandin A2 and 15-deoxy-Delta(12,14)-prostaglandin J2. Increasing reaction pH from 7.4 to 8.9 enhanced apparent second-order rate constants for the thiol reaction with OA-NO2 and LNO2, showing dependence on the thiolate anion of GSH for reactivity. Rates of nitroalkene reaction with thiols decreased as the pKa of target thiols increased. Increasing concentrations of the detergent octyl-beta-d-glucopyranoside decreased rates of nitroalkene reaction with GSH, indicating that the organization of nitro-fatty acids into micellar or membrane structures can limit Michael reactivity with more polar nucleophilic targets. In aggregate, these results reveal that the reversible adduction of thiols by nitro-fatty acids is a mechanism for reversible post-translational regulation of protein function by nitro-fatty acids.
Figures
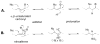




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

