Prostamides (prostaglandin-ethanolamides) and their pharmacology
- PMID: 17721551
- PMCID: PMC2241799
- DOI: 10.1038/sj.bjp.0707434
Prostamides (prostaglandin-ethanolamides) and their pharmacology
Abstract
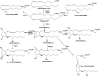
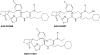
The prostamides are part of a large and continually expanding series of pharmacologically unique neutral lipids. They are COX-2 derived oxidation products of the endocannabinoid/endovanniloid anandamide. Prostamide pharmacology is unique and, as in the case of the endocannabinoids anandamide and 2-arachidonylglycerol, bears little resemblance to that of the corresponding free acids. By virtue of its close relationship to the anti-glaucoma drug bimatoprost, prostamide F(2alpha) has received the greatest research attention. Prostamide F(2alpha) and bimatoprost effects appear independent of prostanoid FP receptor activation, according to a litany of agonist studies. Studies involving freshly isolated and separate feline iridial smooth muscle cells revealed that bimatoprost and FP receptor agonists stimulated different cells, without exception. This suggests the existence of receptors that preferentially recognize prostamide F(2alpha). The recent discovery of prostamide antagonists has provided further support for prostamide receptors as discrete entities. The prototypical prostamide antagonists, AGN 204396 and 7, blocked the effects of prostamide F(2alpha) and bimatoprost but not those of PGF(2alpha) and FP receptor agonists in the feline iris. Second generation more potent prostamide antagonists, such as AGN 211334, should allow the role of prostamides in health and disease to be elucidated. From the therapeutics standpoint, the prostamide F(2alpha) analogue bimatoprost is the most efficacious ocular hypotensive agent currently available for the treatment of glaucoma.
Figures


References
-
- Alm A, Schoenfelder J, McDermott J. A 5-year, multicenter, open-label, safety study of adjunctive latanoprost therapy for glaucoma. Arch Ophthalmol. 2004;122:957–965. - PubMed
-
- Berglund BA, Boring DL, Howlett AC. Investigation of structural analogs of prostaglandin amides for binding to and activation of CB1 and CB2 cannabinoid receptors in rat brain and human tonsils. Adv Exp Med Biol. 1999;469:527–533. - PubMed
-
- Brubaker RF, Schoff EO, Nau CB, Carpenter SP, Chen K, Van Den Burgh AM. Effect of AGN 192024, a new ocular hypotensive agent, on aqueous dynamics. Am J Ophthalmol. 2001;131:19–24. - PubMed
-
- Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61:29–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

