Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI)
- PMID: 17721781
- PMCID: PMC2150634
- DOI: 10.1007/s00262-007-0380-6
Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI)
Abstract
The Cancer Vaccine Consortium of the Sabin Vaccine Institute (CVC/SVI) is conducting an ongoing large-scale immune monitoring harmonization program through its members and affiliated associations. This effort was brought to life as an external validation program by conducting an international Elispot proficiency panel with 36 laboratories in 2005, and was followed by a second panel with 29 participating laboratories in 2006 allowing for application of learnings from the first panel. Critical protocol choices, as well as standardization and validation practices among laboratories were assessed through detailed surveys. Although panel participants had to follow general guidelines in order to allow comparison of results, each laboratory was able to use its own protocols, materials and reagents. The second panel recorded an overall significantly improved performance, as measured by the ability to detect all predefined responses correctly. Protocol choices and laboratory practices, which can have a dramatic effect on the overall assay outcome, were identified and lead to the following recommendations: (A) Establish a laboratory SOP for Elispot testing procedures including (A1) a counting method for apoptotic cells for determining adequate cell dilution for plating, and (A2) overnight rest of cells prior to plating and incubation, (B) Use only pre-tested serum optimized for low background: high signal ratio, (C) Establish a laboratory SOP for plate reading including (C1) human auditing during the reading process and (C2) adequate adjustments for technical artifacts, and (D) Only allow trained personnel, which is certified per laboratory SOPs to conduct assays. Recommendations described under (A) were found to make a statistically significant difference in assay performance, while the remaining recommendations are based on practical experiences confirmed by the panel results, which could not be statistically tested. These results provide initial harmonization guidelines to optimize Elispot assay performance to the immunotherapy community. Further optimization is in process with ongoing panels.
Figures

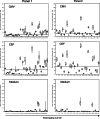
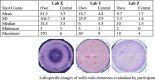

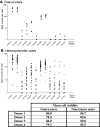
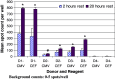
Comment in
-
Toward the harmonization of immune monitoring in clinical trials: quo vadis?Cancer Immunol Immunother. 2008 Mar;57(3):285-8. doi: 10.1007/s00262-007-0379-z. Epub 2007 Aug 25. Cancer Immunol Immunother. 2008. PMID: 17721782 Free PMC article. No abstract available.
-
The CIMT-monitoring panel: a two-step approach to harmonize the enumeration of antigen-specific CD8+ T lymphocytes by structural and functional assays.Cancer Immunol Immunother. 2008 Mar;57(3):289-302. doi: 10.1007/s00262-007-0378-0. Epub 2007 Aug 25. Cancer Immunol Immunother. 2008. PMID: 17721783 Free PMC article.
References
-
- Britten CM, Gouttefangeas C, Schoenmaekers-Welters MJP, Pawelec G, Koch S, Ottensmeier C, Mander A, Walter S, Paschen A, Müller-Berghaus J, Haas I, Mackensen A, Køllgaard T, Thor Straten P, Schmitt M, Giannopoulos K, Maier R, Veelken H, Bertinetti C, Konur A, Huber C, Stevanović S, Wölfel T and Van der Burg SH (2007) The CIMT-monitoring panel: a two-step approach to harmonize the enumeration of antigen-specific CD8+ T lymphocytes by structural and functional assays. Cancer Immunol Immunother (in press). 10.1007/s00262-007-0379-z - PMC - PubMed
-
- Cox J (2005) Conducting a multi-site Elispot proficiency panel. http://www.zellnet.com/february2005/
-
- Cox JH, Ferrari G, Bailer RT, Koup RA. Automating procedures for processing, cryopreservation, storage and manipulation of human peripheral mononuclear cells. J Assoc Lab Automat. 2004;9:16–23. doi: 10.1016/S1535-5535(03)00202-8. - DOI
-
- Cox JH, Ferrari G, Kalams SA, Lopaczynski W, Oden N, D’Souza MP, Elispot Collaborative Study Group Results of an ELISPOT proficiency panel conducted in 11 laboratories participating in international human immunodeficiency virus type 1 vaccine trials. AIDS Res Hum Retroviruses. 2005;21:68–81. doi: 10.1089/aid.2005.21.68. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

