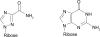Nanomedicines in the treatment of patients with hepatitis C co-infected with HIV--focus on pegylated interferon-alpha
- PMID: 17722274
- PMCID: PMC2676642
- DOI: 10.2147/nano.2006.1.4.399
Nanomedicines in the treatment of patients with hepatitis C co-infected with HIV--focus on pegylated interferon-alpha
Abstract
In immuno-competent individuals, the natural course of chronic hepatitis C virus (HCV) infection is highly variable and 5%-30% of patients develop cirrhosis over 20 years. Co-infection with HCV and human immunodeficiency virus (HIV) is an important prognostic factor and associated with more frequent and accelerated progression to cirrhosis. Until recently HIV/AIDS-related complications were life limiting in patients co-infected with HCV; the introduction of highly active antiretroviral treatment (HAART) and the better prognosis of HIV infection has made HCV-related complications an emerging health problem in HCV/HIV coinfected individuals. Treatment of chronic HCV infection has also evolved since the introduction of interferon-alpha. Recently, introduction of pegylated interferon-alpha (peginterferon-alpha) has resulted in an increase in sustained virus clearance rates of up to 80% in selected genotypes and patient populations. The safety and efficacy of modern anti HCV treatment regimens - based on peginterferon-alpha in combination with ribavirin - was evaluated in 4 controlled trials. Sustained clearance of hepatitis C virus can be achieved in up to 35% of patients with HIV/HCV co-infection, and novel HCV treatment regimens based on peginterferon-alpha have no negative effect on the control of HIV disease. In conclusion, if HIV infection is well controlled and CD4+ cell counts >100/mm3, treatment of chronic hepatitis C with peginterferon in combination with ribavirin is safe and should be given for 48 weeks regardless of the HCV genotype. Introduction of peginterferon-alpha has significantly improved adherence to treatment and treatment efficacy; in particular sustained virologic response in patients with HCV genotype 1 or 4 infection improved, but sustained viral clearance in only 7%-38% of patients infected with genotype I and 4 cannot be the final step in development of effective treatments in patients with HCV/HIV co-infection.
Figures
Similar articles
-
Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial.JAMA. 2004 Dec 15;292(23):2839-48. doi: 10.1001/jama.292.23.2839. JAMA. 2004. PMID: 15598915 Clinical Trial.
-
Management of hepatitis C in HIV infected and other immunocompromised individuals.Trop Gastroenterol. 2006 Jul-Sep;27(3):111-7. Trop Gastroenterol. 2006. PMID: 17310553 Review.
-
Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons.N Engl J Med. 2004 Jul 29;351(5):451-9. doi: 10.1056/NEJMoa032653. N Engl J Med. 2004. PMID: 15282352 Free PMC article. Clinical Trial.
-
Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients.N Engl J Med. 2004 Jul 29;351(5):438-50. doi: 10.1056/NEJMoa040842. N Engl J Med. 2004. PMID: 15282351 Clinical Trial.
-
Treatment of chronic hepatitis C in human immunodeficiency virus/hepatitis C virus-coinfected patients in the era of pegylated interferon and ribavirin.Semin Liver Dis. 2005 Feb;25(1):33-51. doi: 10.1055/s-2005-864780. Semin Liver Dis. 2005. PMID: 15731996 Review.
Cited by
-
Application of nanotechnologies for improved immune response against infectious diseases in the developing world.Adv Drug Deliv Rev. 2010 Mar 18;62(4-5):378-93. doi: 10.1016/j.addr.2009.11.011. Epub 2009 Nov 14. Adv Drug Deliv Rev. 2010. PMID: 19922750 Free PMC article. Review.
-
Management of hepatitis C virus infection in HIV/HCV co-infected patients: clinical review.World J Gastroenterol. 2009 Aug 14;15(30):3713-24. doi: 10.3748/wjg.15.3713. World J Gastroenterol. 2009. PMID: 19673011 Free PMC article. Review.
References
-
- Abonyi ME, Lakatos PL. Ribavirin in the treatment of hepatitis C. Anticancer Res. 2005;25:1315–20. - PubMed
-
- Akuta N, Suzuki F, Suzuki Y, et al. Long-term follow-up of interferon monotherapy in 454 consecutive naive patients infected with hepatitis C virus: multi-course interferon therapy may reduce the risk of hepatocellular carcinoma and increase survival. Scand J Gastroenterol. 2005;40:688–96. - PubMed
-
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–9. - PubMed
-
- Belle SH, Beringer KC, Detre KM. Recent findings concerning liver transplantation in the United States. In: Terasaki PI, Cecka JM, editors. Clinical transplants. Los Angeles: UCLA Tissue Typing Laboratory; 1996. pp. 15–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials