Megestrol acetate in cachexia and anorexia
- PMID: 17722275
- PMCID: PMC2676640
- DOI: 10.2147/nano.2006.1.4.411
Megestrol acetate in cachexia and anorexia
Abstract
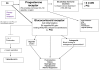
The aim is to review major clinical trials that have used megestrol acetate (MA) in the treatment of cachexia across several disease states. A review of general usage and potential side-effects are discussed. A theory that the newly approved nanocrystal formation of MA can better deliver this potent medication for treatment will also be reviewed.
Figures
References
-
- Alakhov V, Pietrzynski G, Patel K, et al. Pluronic block copolymers and Pluronic poly(acrylic acid) microgels in oral delivery of megestrol acetate. J Pharm Pharmacol. 2004;56:1233–41. - PubMed
-
- Barak V, Schwartz A, Kalickman I, et al. Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines. Am J Med. 1998;104:40–7. - PubMed
-
- Bennett RG.2003Megestrol complications Chest 123309–10.author reply 310. - PubMed
-
- Berenstein E, Ortiz Z. Megestrol acetate for the treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2005:CD004310. - PubMed
-
- Bojar H, Maar K, Staib W. The endocrine background of human renal cell carcinoma. IV. Glucocorticoid receptors as possible mediators of progestogen action. Urol Int. 1979;34:330–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

