Inhibition of the Mycobacterium tuberculosis enoyl acyl carrier protein reductase InhA by arylamides
- PMID: 17723305
- PMCID: PMC2020492
- DOI: 10.1016/j.bmc.2007.08.013
Inhibition of the Mycobacterium tuberculosis enoyl acyl carrier protein reductase InhA by arylamides
Abstract
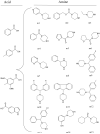
InhA, the enoyl acyl carrier protein reductase (ENR) from Mycobacterium tuberculosis, is one of the key enzymes involved in the type II fatty acid biosynthesis pathway of M. tuberculosis. We report here the discovery, through high-throughput screening, of a series of arylamides as a novel class of potent InhA inhibitors. These direct InhA inhibitors require no mycobacterial enzymatic activation and thus circumvent the resistance mechanism to antitubercular prodrugs such as INH and ETA that is most commonly observed in drug-resistant clinical isolates. The crystal structure of InhA complexed with one representative inhibitor reveals the binding mode of the inhibitor within the InhA active site. Further optimization through a microtiter synthesis strategy followed by in situ activity screening led to the discovery of a potent InhA inhibitor with in vitro IC(50)=90 nM, representing a 34-fold potency improvement over the lead compound.
Figures

Similar articles
-
Development of Mycobacterium tuberculosis Enoyl Acyl Reductase (InhA) Inhibitors: A Mini-Review.Mini Rev Med Chem. 2025;25(3):219-233. doi: 10.2174/0113895575309785240902102421. Mini Rev Med Chem. 2025. PMID: 39301902 Review.
-
Pyrrolidine carboxamides as a novel class of inhibitors of enoyl acyl carrier protein reductase from Mycobacterium tuberculosis.J Med Chem. 2006 Oct 19;49(21):6308-23. doi: 10.1021/jm060715y. J Med Chem. 2006. PMID: 17034137 Free PMC article.
-
Structure-based design of a novel class of potent inhibitors of InhA, the enoyl acyl carrier protein reductase from Mycobacterium tuberculosis: a computer modelling approach.Chem Biol Drug Des. 2008 Nov;72(5):444-9. doi: 10.1111/j.1747-0285.2008.00722.x. Chem Biol Drug Des. 2008. PMID: 19012578
-
A slow, tight binding inhibitor of InhA, the enoyl-acyl carrier protein reductase from Mycobacterium tuberculosis.J Biol Chem. 2010 May 7;285(19):14330-7. doi: 10.1074/jbc.M109.090373. Epub 2010 Mar 3. J Biol Chem. 2010. PMID: 20200152 Free PMC article.
-
Recent Advances and Structural Features of Enoyl-ACP Reductase Inhibitors of Mycobacterium tuberculosis.Arch Pharm (Weinheim). 2016 Nov;349(11):817-826. doi: 10.1002/ardp.201600186. Epub 2016 Oct 24. Arch Pharm (Weinheim). 2016. PMID: 27775177 Review.
Cited by
-
Structure-Based Design and in Silico Screening of Virtual Combinatorial Library of Benzamides Inhibiting 2-trans Enoyl-Acyl Carrier Protein Reductase of Mycobacterium tuberculosis with Favorable Predicted Pharmacokinetic Profiles.Int J Mol Sci. 2019 Sep 24;20(19):4730. doi: 10.3390/ijms20194730. Int J Mol Sci. 2019. PMID: 31554227 Free PMC article.
-
The pathogenic mechanism of Mycobacterium tuberculosis: implication for new drug development.Mol Biomed. 2022 Dec 22;3(1):48. doi: 10.1186/s43556-022-00106-y. Mol Biomed. 2022. PMID: 36547804 Free PMC article. Review.
-
Discovery of novel and potent InhA direct inhibitors by ensemble docking-based virtual screening and biological assays.J Comput Aided Mol Des. 2023 Dec;37(12):695-706. doi: 10.1007/s10822-023-00530-4. Epub 2023 Aug 29. J Comput Aided Mol Des. 2023. PMID: 37642861
-
In Silico Repositioning of Cannabigerol as a Novel Inhibitor of the Enoyl Acyl Carrier Protein (ACP) Reductase (InhA).Molecules. 2019 Jul 15;24(14):2567. doi: 10.3390/molecules24142567. Molecules. 2019. PMID: 31311157 Free PMC article.
-
Development of Mycobacterium tuberculosis Enoyl Acyl Reductase (InhA) Inhibitors: A Mini-Review.Mini Rev Med Chem. 2025;25(3):219-233. doi: 10.2174/0113895575309785240902102421. Mini Rev Med Chem. 2025. PMID: 39301902 Review.
References
-
- Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um KS, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. - PubMed
-
- Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. - PubMed
-
- Escalante P, Ramaswamy S, Sanabria H, Soini H, Pan X, et al. Genotypic characterization of drug-resistant Mycobacterium tuberculosis isolates from Peru. Tuber Lung Dis. 1998;79:111–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

