Novel potentiometric and optical silver ion-selective sensors with subnanomolar detection limits
- PMID: 17723454
- PMCID: PMC2883728
- DOI: 10.1016/j.aca.2006.05.009
Novel potentiometric and optical silver ion-selective sensors with subnanomolar detection limits
Abstract
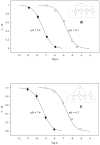
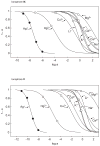
Ten Ag+-selective ionophores have been characterized in terms of their potentiometric selectivities and complex formation constants in solvent polymeric membranes. The compounds with pi-coordination show much weaker interactions than those with thioether or thiocarbamate groups as the coordinating sites. Long-term studies with the best ionophores show that the lower detection limit of the best Ag+ sensors can be maintained in the subnanomolar range for at least 1 month. The best ionophores have also been characterized in fluorescent microspheres. The so far best lower detection limits of 3 x 10(-11) M (potentiometrically) and 2 x 10(-11) M Ag+ (optically) are found with bridged thiacalixarenes.
Figures

Similar articles
-
Highly selective detection of silver in the low ppt range with ion-selective electrodes based on ionophore-doped fluorous membranes.Anal Chem. 2010 Sep 15;82(18):7634-40. doi: 10.1021/ac1013767. Anal Chem. 2010. PMID: 20799720 Free PMC article.
-
Potentiometric sensors based on fluorous membranes doped with highly selective ionophores for carbonate.J Am Chem Soc. 2011 Dec 28;133(51):20869-77. doi: 10.1021/ja207680e. Epub 2011 Dec 2. J Am Chem Soc. 2011. PMID: 22070518 Free PMC article.
-
New calix[4]arene derivatives as ionophores in polymeric membrane electrodes for Ag(I): comparative selectivity studies and detection of DNA hybridization.Talanta. 2013 Feb 15;105:1-7. doi: 10.1016/j.talanta.2012.11.046. Epub 2012 Nov 27. Talanta. 2013. PMID: 23597979
-
Reliable environmental trace heavy metal analysis with potentiometric ion sensors - reality or a distant dream.Environ Pollut. 2021 Nov 15;289:117882. doi: 10.1016/j.envpol.2021.117882. Epub 2021 Jul 30. Environ Pollut. 2021. PMID: 34364114 Review.
-
Membrane sensors based on Schiff bases as chelating ionophores--a review.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Nov 11;132:854-70. doi: 10.1016/j.saa.2014.04.176. Epub 2014 Jun 3. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24947440 Review.
Cited by
-
Disposable Multi-Walled Carbon Nanotubes-Based Plasticizer-Free Solid-Contact Pb2+-Selective Electrodes with a Sub-PPB Detection Limit †.Sensors (Basel). 2019 Jun 4;19(11):2550. doi: 10.3390/s19112550. Sensors (Basel). 2019. PMID: 31167473 Free PMC article.
-
Potentiometry at trace levels in confined samples: ion-selective electrodes with subfemtomole detection limits.J Am Chem Soc. 2006 Jun 28;128(25):8154-5. doi: 10.1021/ja0625780. J Am Chem Soc. 2006. PMID: 16787077 Free PMC article.
-
Modern potentiometry.Angew Chem Int Ed Engl. 2007;46(30):5660-8. doi: 10.1002/anie.200605068. Angew Chem Int Ed Engl. 2007. PMID: 17457791 Free PMC article. Review.
-
Modern Directions for Potentiometric Sensors.J Braz Chem Soc. 2008 Jan 1;19(4):621-629. doi: 10.1590/S0103-50532008000400003. J Braz Chem Soc. 2008. PMID: 19890473 Free PMC article.
-
Potentiometric detection of DNA hybridization using enzyme-induced metallization and a silver ion selective electrode.Anal Chem. 2009 Dec 15;81(24):10007-12. doi: 10.1021/ac9018507. Anal Chem. 2009. PMID: 19908886 Free PMC article.
References
-
- Lai MT, Shih JS. Analyst. 1986;111:891.
-
- Bühlmann P, Pretsch E, Bakker E. Chem Rev. 1998;98:1593. - PubMed
-
- Bakker E, Bühlmann P, Pretsch E. Electroanalysis. 1999;11:915.
-
- Bryce MR, Johnston B, Kataky R, Tóth K. Analyst. 2000;125:861.
-
- Xu D, Katsu T. Anal Chim Acta. 2001;443:235.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

