Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation
- PMID: 17724027
- PMCID: PMC2267748
- DOI: 10.1074/jbc.M703091200
Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation
Abstract
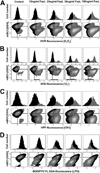
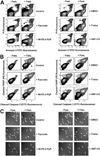
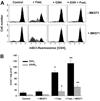
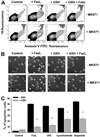
Changes in the intracellular redox environment of cells have been reported to be critical for the activation of apoptotic enzymes and the progression of programmed cell death. Glutathione (GSH) depletion is an early hallmark observed in apoptosis, and we have demonstrated that GSH efflux during death receptor-mediated apoptosis occurs via a GSH transporter. We now evaluate the relationship between GSH depletion, the generation of reactive oxygen species (ROS), and the progression of apoptosis. Simultaneous single cell analysis of changes in GSH content and ROS formation by multiparametric FACS revealed that loss of intracellular GSH was paralleled by the generation of different ROS including hydrogen peroxide, superoxide anion, hydroxyl radical, and lipid peroxides. However, inhibition of ROS formation by a variety of antioxidants showed that GSH loss was independent from the generation of ROS. Furthermore, GSH depletion was observed to be necessary for ROS generation. Interestingly, high extracellular thiol concentration (GSH and N-acetyl-cysteine) inhibited apoptosis, whereas, inhibition of ROS generation by other non-thiol antioxidants was ineffective in preventing cell death. Finally, GSH depletion was shown to be a necessary for the progression of apoptosis activated by both extrinsic and intrinsic signaling pathways. These results document a necessary and critical role for GSH loss in apoptosis and clearly uncouple for the first time GSH depletion from ROS formation.
Figures






Similar articles
-
Pyrogallol as a glutathione depletor induces apoptosis in HeLa cells.Int J Mol Med. 2008 Jun;21(6):721-30. Int J Mol Med. 2008. PMID: 18506365
-
Arsenic trioxide induces human pulmonary fibroblast cell death via increasing ROS levels and GSH depletion.Oncol Rep. 2012 Aug;28(2):749-57. doi: 10.3892/or.2012.1852. Epub 2012 Jun 6. Oncol Rep. 2012. PMID: 22684917
-
Roles of oxidative stress and glutathione depletion in JP-8 jet fuel-induced apoptosis in rat lung epithelial cells.Toxicol Appl Pharmacol. 2002 Apr 15;180(2):92-9. doi: 10.1006/taap.2002.9350. Toxicol Appl Pharmacol. 2002. PMID: 11969376
-
Glutathione efflux and cell death.Antioxid Redox Signal. 2012 Dec 15;17(12):1694-713. doi: 10.1089/ars.2012.4553. Epub 2012 Jul 16. Antioxid Redox Signal. 2012. PMID: 22656858 Free PMC article. Review.
-
Reactive oxygen species, cellular redox systems, and apoptosis.Free Radic Biol Med. 2010 Mar 15;48(6):749-62. doi: 10.1016/j.freeradbiomed.2009.12.022. Epub 2010 Jan 4. Free Radic Biol Med. 2010. PMID: 20045723 Free PMC article. Review.
Cited by
-
Volume-sensitive anion channels mediate osmosensitive glutathione release from rat thymocytes.PLoS One. 2013;8(1):e55646. doi: 10.1371/journal.pone.0055646. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383255 Free PMC article.
-
The glutathione system: a new drug target in neuroimmune disorders.Mol Neurobiol. 2014 Dec;50(3):1059-84. doi: 10.1007/s12035-014-8705-x. Epub 2014 Apr 22. Mol Neurobiol. 2014. PMID: 24752591 Review.
-
Selective Killing Effects of Cold Atmospheric Pressure Plasma with NO Induced Dysfunction of Epidermal Growth Factor Receptor in Oral Squamous Cell Carcinoma.PLoS One. 2016 Feb 26;11(2):e0150279. doi: 10.1371/journal.pone.0150279. eCollection 2016. PLoS One. 2016. PMID: 26919318 Free PMC article.
-
Injectable thermo-sensitive hydrogel loaded hollow copper sulfide nanoparticles for ROS burst in TME and effective tumor treatment.Front Bioeng Biotechnol. 2023 May 2;11:1191014. doi: 10.3389/fbioe.2023.1191014. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37200848 Free PMC article.
-
Roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma.Life Sci. 2013 Jan 17;92(1):3-9. doi: 10.1016/j.lfs.2012.10.009. Epub 2012 Oct 30. Life Sci. 2013. PMID: 23123447 Free PMC article.
References
-
- Green DR. Immunol. Rev. 2003;193:5–9. - PubMed
-
- Fadeel B, Orrenius S. J. Intern. Med. 2005;258:479–517. - PubMed
-
- Brunner T, Wasem C, Torgler R, Cima I, Jakob S, Corazza N. Semin. Immunol. 2003;15:167–176. - PubMed
-
- Bortner CD, Cidlowski JA. Cell Death Differ. 2002;9:1307–1310. - PubMed
-
- Franco R, Bortner CD, Cidlowski JA. J. Membr. Biol. 2006;209:43–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

