Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli
- PMID: 17724068
- PMCID: PMC2168307
- DOI: 10.1128/IAI.00922-07
Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli
Abstract
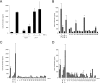
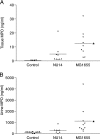
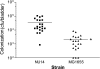
Uropathogenic Escherichia coli (UPEC), the most frequent cause of urinary tract infection (UTI), is associated with an inflammatory response which includes the induction of cytokine/chemokine secretion by urothelial cells and neutrophil recruitment to the bladder. Recent studies indicate, however, that UPEC can evade the early activation of urothelial innate immune response in vitro. In this study, we report that infection with the prototypic UPEC strain NU14 suppresses tumor necrosis factor alpha (TNF-alpha)-mediated interleukin-8 (CXCL-8) and interleukin-6 (CXCL-6) secretion from urothelial cell cultures compared to infection with a type 1 piliated E. coli K-12 strain. Furthermore, examination of a panel of clinical E. coli isolates revealed that 15 of 17 strains also possessed the ability to suppress cytokine secretion. In a murine model of UTI, NU14 infection resulted in diminished levels of mRNAs encoding keratinocyte-derived chemokine, macrophage inflammatory peptide 2, and CXCL-6 in the bladder relative to infection with an E. coli K-12 strain. Furthermore, reduced stimulation of inflammatory chemokine production during NU14 infection correlated with decreased levels of bladder and urine myeloperoxidase and increased bacterial colonization. These data indicate that a broad phylogenetic range of clinical E. coli isolates, including UPEC, may evade the activation of innate immune response in the urinary tract, thereby providing a pathogenic advantage.
Figures
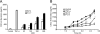

References
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
-
- Anderson, G. G., S. M. Martin, and S. J. Hultgren. 2004. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect. 6:1094-1101. - PubMed
-
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
-
- Backhed, F., L. Meijer, S. Normark, and A. Richter-Dahlfors. 2002. TLR4-dependent recognition of lipopolysaccharide by epithelial cells requires sCD14. Cell. Microbiol. 4:493-501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

