Effects of DNA- and Mycobacterium bovis BCG-based delivery of the Flt3 ligand on protective immunity to Mycobacterium tuberculosis
- PMID: 17724075
- PMCID: PMC2168302
- DOI: 10.1128/IAI.00322-07
Effects of DNA- and Mycobacterium bovis BCG-based delivery of the Flt3 ligand on protective immunity to Mycobacterium tuberculosis
Abstract
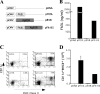
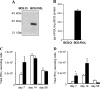
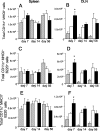
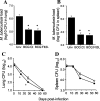
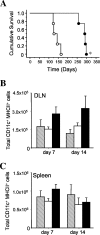
The control of intracellular pathogens such as Mycobacterium tuberculosis is dependent on the activation and maintenance of pathogen-reactive T cells. Dendritic cells (DCs) are the major antigen-presenting cells initiating antimycobacterial T-cell responses in vivo. To investigate if immunization strategies that aim to optimize DC function can improve protective immunity against virulent mycobacterial infection, we exploited the ability of the hematopoietic growth factor Fms-like tyrosine kinase 3 ligand (Flt3L) to expand the number of DCs in vivo. A DNA fusion of the genes encoding murine Flt3L and M. tuberculosis antigen 85B stimulated enhanced gamma interferon (IFN-gamma) release by T cells and provided better protection against virulent M. tuberculosis than DNA encoding the single components. Vaccination of mice with a recombinant Mycobacterium bovis BCG strain secreting Flt3L (BCG:Flt3L) led to early expansion of DCs compared to immunization with BCG alone, and this effect was associated with increased stimulation of BCG-reactive IFN-gamma-secreting T cells. BCG and BCG:Flt3L provided similar protective efficacies against low-dose aerosol M. tuberculosis; however, immunization of immunodeficient mice revealed that BCG:Flt3L was markedly less virulent than conventional BCG. These results demonstrate the potential of in vivo targeting of DCs to improve antimycobacterial vaccine efficacy.
Figures
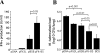
Similar articles
-
Recombinant BCG coexpressing Ag85B, ESAT-6 and mouse-IFN-gamma confers effective protection against Mycobacterium tuberculosis in C57BL/6 mice.FEMS Immunol Med Microbiol. 2007 Dec;51(3):480-7. doi: 10.1111/j.1574-695X.2007.00322.x. Epub 2007 Oct 4. FEMS Immunol Med Microbiol. 2007. PMID: 17919299
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Vaccination with a Sindbis virus-based DNA vaccine expressing antigen 85B induces protective immunity against Mycobacterium tuberculosis.Infect Immun. 2005 Nov;73(11):7727-35. doi: 10.1128/IAI.73.11.7727-7735.2005. Infect Immun. 2005. PMID: 16239577 Free PMC article.
-
Immunogenicity and protective efficacy conferred by a novel recombinant Mycobacterium bovis bacillus Calmette-Guérin strain expressing interleukin-12p70 of human cytokine and Ag85A of Mycobacterium tuberculosis fusion protein.Scand J Immunol. 2013 Dec;78(6):497-506. doi: 10.1111/sji.12116. Scand J Immunol. 2013. PMID: 24283772
-
Prospects for new vaccines against tuberculosis.Biotechniques. 2002 Nov;33(5):1098, 1100, 1102. doi: 10.2144/02335dd01. Biotechniques. 2002. PMID: 12449388 Review. No abstract available.
Cited by
-
Recombinant BCG to Enhance Its Immunomodulatory Activities.Vaccines (Basel). 2022 May 23;10(5):827. doi: 10.3390/vaccines10050827. Vaccines (Basel). 2022. PMID: 35632582 Free PMC article. Review.
-
The nasal dendritic cell-targeting Flt3 ligand as a safe adjuvant elicits effective protection against fatal pneumococcal pneumonia.Infect Immun. 2011 Jul;79(7):2819-28. doi: 10.1128/IAI.01360-10. Epub 2011 May 2. Infect Immun. 2011. PMID: 21536790 Free PMC article.
-
Immune Responses to Bacillus Calmette-Guérin Vaccination: Why Do They Fail to Protect against Mycobacterium tuberculosis?Front Immunol. 2017 Apr 5;8:407. doi: 10.3389/fimmu.2017.00407. eCollection 2017. Front Immunol. 2017. PMID: 28424703 Free PMC article. Review.
-
Pathological and protective roles of dendritic cells in Mycobacterium tuberculosis infection: Interaction between host immune responses and pathogen evasion.Front Cell Infect Microbiol. 2022 Jul 28;12:891878. doi: 10.3389/fcimb.2022.891878. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35967869 Free PMC article. Review.
-
FMS-like tyrosine kinase 3 ligand treatment of mice aggravates acute lung injury in response to Streptococcus pneumoniae: role of pneumolysin.Infect Immun. 2012 Dec;80(12):4281-90. doi: 10.1128/IAI.00854-12. Epub 2012 Sep 24. Infect Immun. 2012. PMID: 23006850 Free PMC article.
References
-
- Alaniz, R. C., S. Sandall, E. K. Thomas, and C. B. Wilson. 2004. Increased dendritic cell numbers impair protective immunity to intracellular bacteria despite augmenting antigen-specific CD8+ T lymphocyte responses. J. Immunol. 172:3725-3735. - PubMed
-
- Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. - PubMed
-
- Baek, K. M., S. Y. Ko, M. Lee, J. S. Lee, J. O. Kim, H. J. Ko, J. W. Lee, S. H. Lee, S. N. Cho, and C. Y. Kang. 2003. Comparative analysis of effects of cytokine gene adjuvants on DNA vaccination against Mycobacterium tuberculosis heat shock protein 65. Vaccine 21:3684-3689. - PubMed
-
- Bhatt, K., S. P. Hickman, and P. Salgame. 2004. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. J. Immunol. 172:2748-2751. - PubMed
-
- Britton, W. J., and U. Palendira. 2003. Improving vaccines against tuberculosis. Immunol. Cell Biol. 81:34-45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

