G protein-coupled receptor Ca2+-linked mitochondrial reactive oxygen species are essential for endothelial/leukocyte adherence
- PMID: 17724077
- PMCID: PMC2169045
- DOI: 10.1128/MCB.00493-07
G protein-coupled receptor Ca2+-linked mitochondrial reactive oxygen species are essential for endothelial/leukocyte adherence
Abstract
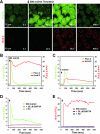
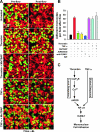
Receptor-mediated signaling is commonly associated with multiple functions, including the production of reactive oxygen species. However, whether mitochondrion-derived superoxide (mROS) contributes directly to physiological signaling is controversial. Here we demonstrate a previously unknown mechanism in which physiologic Ca(2+)-evoked mROS production plays a pivotal role in endothelial cell (EC) activation and leukocyte firm adhesion. G protein-coupled receptor (GPCR) and tyrosine kinase-mediated inositol 1,4,5-trisphosphate-dependent mitochondrial Ca(2+) uptake resulted in NADPH oxidase-independent mROS production. However, GPCR-linked mROS production did not alter mitochondrial function or trigger cell death but rather contributed to activation of NF-kappaB and leukocyte adhesion via the EC induction of intercellular adhesion molecule 1. Dismutation of mROS by manganese superoxide dismutase overexpression and a cell-permeative superoxide dismutase mimetic ablated NF-kappaB transcriptional activity and facilitated leukocyte detachment from the endothelium under simulated circulation following GPCR- but not cytokine-induced activation. These results demonstrate that mROS is the downstream effector molecule that translates receptor-mediated Ca(2+) signals into proinflammatory signaling and leukocyte/EC firm adhesion.
Figures




Similar articles
-
Superoxide dismutase inhibits the expression of vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 induced by tumor necrosis factor-alpha in human endothelial cells through the JNK/p38 pathways.Arterioscler Thromb Vasc Biol. 2005 Feb;25(2):334-40. doi: 10.1161/01.ATV.0000152114.00114.d8. Epub 2004 Dec 2. Arterioscler Thromb Vasc Biol. 2005. PMID: 15576639
-
HMG-CoA reductase inhibitors reduce nicotine-induced expression of cellular adhesion molecules in cultured human coronary endothelial cells.J Vasc Res. 2007;44(6):460-70. doi: 10.1159/000106464. Epub 2007 Jul 26. J Vasc Res. 2007. PMID: 17657162
-
Mechanisms of modulation of brain microvascular endothelial cells function by thrombin.Brain Res. 2017 Feb 15;1657:167-175. doi: 10.1016/j.brainres.2016.12.011. Epub 2016 Dec 18. Brain Res. 2017. PMID: 27998795 Free PMC article.
-
Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance.Front Cell Infect Microbiol. 2017 Aug 25;7:373. doi: 10.3389/fcimb.2017.00373. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28890882 Free PMC article. Review.
-
Signaling in leukocyte transendothelial migration.Arterioscler Thromb Vasc Biol. 2004 May;24(5):824-33. doi: 10.1161/01.ATV.0000122854.76267.5c. Epub 2004 Feb 19. Arterioscler Thromb Vasc Biol. 2004. PMID: 14976004 Review.
Cited by
-
Update on vascular endothelial Ca(2+) signalling: A tale of ion channels, pumps and transporters.World J Biol Chem. 2012 Jul 26;3(7):127-58. doi: 10.4331/wjbc.v3.i7.127. World J Biol Chem. 2012. PMID: 22905291 Free PMC article.
-
Overexpression of hexokinase 2 reduces mitochondrial calcium overload in coronary endothelial cells of type 2 diabetic mice.Am J Physiol Cell Physiol. 2018 Jun 1;314(6):C732-C740. doi: 10.1152/ajpcell.00350.2017. Epub 2018 Mar 7. Am J Physiol Cell Physiol. 2018. PMID: 29513568 Free PMC article.
-
Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis.Free Radic Biol Med. 2010 Jan 15;48(2):306-17. doi: 10.1016/j.freeradbiomed.2009.11.001. Epub 2009 Nov 5. Free Radic Biol Med. 2010. PMID: 19897030 Free PMC article.
-
Execution of superoxide-induced cell death by the proapoptotic Bcl-2-related proteins Bid and Bak.Mol Cell Biol. 2009 Jun;29(11):3099-112. doi: 10.1128/MCB.01845-08. Epub 2009 Mar 30. Mol Cell Biol. 2009. PMID: 19332555 Free PMC article.
-
P2Y2-P2X7 receptors cross-talk in primed mesenteric endothelial cells upregulates NF-κB signaling favoring mononuclear cell adhesion in schistosomiasis.Front Immunol. 2024 Jan 4;14:1328897. doi: 10.3389/fimmu.2023.1328897. eCollection 2023. Front Immunol. 2024. PMID: 38239348 Free PMC article.
References
-
- Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12: 141-179. - PubMed
-
- Bernardi, P., L. Scorrano, R. Colonna, V. Petronilli, and F. Di Lisa. 1999. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 264: 687-701. - PubMed
-
- Berridge, M. J., M. D. Bootman, and P. Lipp. 1998. Calcium—a life and death signal. Nature 395: 645-648. - PubMed
-
- Berridge, M. J., M. D. Bootman, and H. L. Roderick. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4: 517-529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
