Conditional deletion of activating protein 2alpha (AP-2alpha) in the developing retina demonstrates non-cell-autonomous roles for AP-2alpha in optic cup development
- PMID: 17724084
- PMCID: PMC2169054
- DOI: 10.1128/MCB.00687-07
Conditional deletion of activating protein 2alpha (AP-2alpha) in the developing retina demonstrates non-cell-autonomous roles for AP-2alpha in optic cup development
Abstract
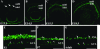
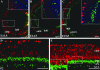
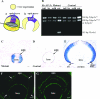
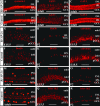
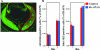
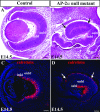
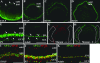
Activating protein 2alpha (AP-2alpha) is known to be expressed in the retina, and AP-2alpha-null mice exhibit defects in the developing optic cup, including patterning of the neural retina (NR) and a replacement of the dorsal retinal pigmented epithelium (RPE) with NR. In this study, we analyzed the temporal and spatial retinal expression patterns of AP-2alpha and created a conditional deletion of AP-2alpha in the developing retina. AP-2alpha exhibited a distinct expression pattern in the developing inner nuclear layer of the retina, and colocalization studies indicated that AP-2alpha was exclusively expressed in postmitotic amacrine cell populations. Targeted deletion of AP-2alpha in the developing retina did not result in observable retinal defects. Further examination of AP-2alpha-null mutants revealed that the severity of the RPE defect was variable and, although defects in retinal lamination occur at later embryonic stages, earlier stages showed normal lamination and expression of markers for amacrine and ganglion cells. Together, these data demonstrate that, whereas AP-2alpha alone does not play an intrinsic role in retinogenesis, it has non-cell-autonomous effects on optic cup development. Additional expression analyses showed that multiple AP-2 proteins are present in the developing retina, which will be important to future studies.
Figures




References
-
- Akagawa, K., M. Takada, H. Hayashi, and K. Uyemura. 1990. Calcium- and voltage-dependent potassium channel in the rat retinal amacrine cells identified in vitro using a cell type-specific monoclonal antibody. Brain Res. 518: 1-5. - PubMed
-
- Baumer, N., T. Marquardt, A. Stoykova, R. Ashery-Padan, K. Chowdhury, and P. Gruss. 2002. Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development 129: 4535-4545. - PubMed
-
- Bisgrove, D. A., and R. Godbout. 1999. Differential expression of AP-2α and AP-2β in the developing chick retina: repression of R-FABP promoter activity by AP-2. Dev. Dyn. 214: 195-206. - PubMed
-
- Bosher, J. M., N. F. Totty, J. J. Hsuan, T. Williams, and H. C. Hurst. 1996. A family of AP-2 proteins regulates c-erbB-2 expression in mammary carcinoma. Oncogene 13: 1701-1707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
