DLGH1 is a negative regulator of T-lymphocyte proliferation
- PMID: 17724087
- PMCID: PMC2169038
- DOI: 10.1128/MCB.00439-07
DLGH1 is a negative regulator of T-lymphocyte proliferation
Abstract
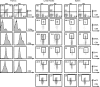
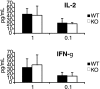
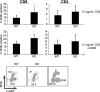
Discs large homolog 1 (DLGH1), a founding member of the membrane-associated guanylate kinase family of proteins containing PostSynaptic Density-95/Discs large/Zona Occludens-1 domains, is an ortholog of the Drosophila tumor suppressor gene Discs large. In the mammalian embryo, DLGH1 is essential for normal urogenital morphogenesis and the development of skeletal and epithelial structures. Recent reports also indicate that DLGH1 may be a critical mediator of signals triggered by the antigen receptor complex in T lymphocytes by functioning as a scaffold coordinating the activities of T-cell receptor (TCR) signaling proteins at the immune synapse. However, it remains unclear if DLGH1 functions to enhance or attenuate signals emanating from the TCR. Here, we used Dlgh1 gene-targeted mice to determine the requirement for DLGH1 in T-cell development and activation. Strikingly, while all major subsets of T cells appear to undergo normal thymic development in the absence of DLGH1, peripheral lymph node Dlgh1(-/-) T cells show a hyper-proliferative response to TCR-induced stimulation. These data indicate that, consistent with the known function of Discs large proteins as tumor suppressors and attenuators of cell division, in T lymphocytes, DLGH1 functions as a negative regulator of TCR-induced proliferative responses.
Figures




References
-
- Bachmaier, K., C. Krawczyk, I. Kozieradzki, Y. Y. Kong, T. Sasaki, A. Oliveira-dos Santos, S. Mariathasan, D. Bouchard, A. Wakeham, A. Itie, J. Le, P. S. Ohashi, I. Sarosi, H. Nishina, S. Lipkowitz, and J. M. Penninger. 2000. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403: 211-216. - PubMed
-
- Betschinger, J., K. Mechtler, and J. A. Knoblich. 2003. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422: 326-330. - PubMed
-
- Caruana, G. 2002. Genetic studies define MAGUK proteins as regulators of epithelial cell polarity. Int. J. Dev. Biol. 46: 511-518. - PubMed
-
- Dimitratos, S. D., D. F. Woods, D. G. Stathakis, and P. J. Bryant. 1999. Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. Bioessays 21: 912-921. - PubMed
-
- Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature 421: 753-756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
