Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination
- PMID: 17724124
- PMCID: PMC2064549
- DOI: 10.1083/jcb.200705132
Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination
Abstract
Axon-glial interactions are critical for the induction of myelination and the domain organization of myelinated fibers. Although molecular complexes that mediate these interactions in the nodal region are known, their counterparts along the internode are poorly defined. We report that neurons and Schwann cells express distinct sets of nectin-like (Necl) proteins: axons highly express Necl-1 and -2, whereas Schwann cells express Necl-4 and lower amounts of Necl-2. These proteins are strikingly localized to the internode, where Necl-1 and -2 on the axon are directly apposed by Necl-4 on the Schwann cell; all three proteins are also enriched at Schmidt-Lanterman incisures. Binding experiments demonstrate that the Necl proteins preferentially mediate heterophilic rather than homophilic interactions. In particular, Necl-1 on axons binds specifically to Necl-4 on Schwann cells. Knockdown of Necl-4 by short hairpin RNA inhibits Schwann cell differentiation and subsequent myelination in cocultures. These results demonstrate a key role for Necl-4 in initiating peripheral nervous system myelination and implicate the Necl proteins as mediators of axo-glial interactions along the internode.
Figures
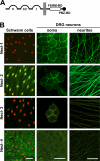
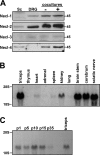



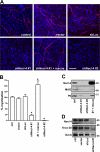


Similar articles
-
Putting the glue in glia: Necls mediate Schwann cell axon adhesion.J Cell Biol. 2007 Aug 27;178(5):721-3. doi: 10.1083/jcb.200708019. J Cell Biol. 2007. PMID: 17724116 Free PMC article.
-
Necl-4/Cadm4 recruits Par-3 to the Schwann cell adaxonal membrane.Glia. 2019 May;67(5):884-895. doi: 10.1002/glia.23578. Epub 2018 Dec 26. Glia. 2019. PMID: 30585357 Free PMC article.
-
The 4.1B cytoskeletal protein regulates the domain organization and sheath thickness of myelinated axons.Glia. 2013 Feb;61(2):240-53. doi: 10.1002/glia.22430. Epub 2012 Oct 26. Glia. 2013. PMID: 23109359 Free PMC article.
-
Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation.Glia. 2008 Nov 1;56(14):1491-1497. doi: 10.1002/glia.20753. Glia. 2008. PMID: 18803318 Review.
-
The effect of myelinating Schwann cells on axons.Muscle Nerve. 2001 Apr;24(4):456-66. doi: 10.1002/mus.1027. Muscle Nerve. 2001. PMID: 11268016 Review.
Cited by
-
Cthrc1 is a negative regulator of myelination in Schwann cells.Glia. 2012 Mar;60(3):393-403. doi: 10.1002/glia.22273. Glia. 2012. PMID: 22379615 Free PMC article.
-
Differential Contribution of Cadm1-Cadm3 Cell Adhesion Molecules to Peripheral Myelinated Axons.J Neurosci. 2021 Feb 17;41(7):1393-1400. doi: 10.1523/JNEUROSCI.2736-20.2020. Epub 2021 Jan 4. J Neurosci. 2021. PMID: 33397712 Free PMC article.
-
SynCAM, a novel putative tumor suppressor, suppresses growth and invasiveness of glioblastoma.Mol Biol Rep. 2013 Sep;40(9):5469-75. doi: 10.1007/s11033-013-2645-9. Epub 2013 Aug 2. Mol Biol Rep. 2013. PMID: 23907434
-
Downregulating SynCAM and MPP6 expression is associated with ovarian cancer progression.Oncol Lett. 2019 Sep;18(3):2477-2483. doi: 10.3892/ol.2019.10542. Epub 2019 Jun 28. Oncol Lett. 2019. PMID: 31402947 Free PMC article.
-
Homophilic interaction of cell adhesion molecule 3 coordinates retina neuroepithelial cell proliferation.J Cell Biol. 2023 Jun 5;222(6):e202204098. doi: 10.1083/jcb.202204098. Epub 2023 Apr 6. J Cell Biol. 2023. PMID: 37022761 Free PMC article.
References
-
- Arase, N., A. Takeuchi, M. Unno, S. Hirano, T. Yokosuka, H. Arase, and T. Saito. 2005. Heterotypic interaction of CRTAM with Necl2 induces cell adhesion on activated NK cells and CD8+ T cells. Int. Immunol. 17:1227–1237. - PubMed
-
- Biederer, T. 2006. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics. 87:139–150. - PubMed
-
- Biederer, T., Y. Sara, M. Mozhayeva, D. Atasoy, X. Liu, E.T. Kavalali, and T.C. Sudhof. 2002. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 297:1525–1531. - PubMed
-
- Boles, K.S., W. Barchet, T. Diacovo, M. Cella, and M. Colonna. 2005. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood. 106:779–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

