Thymic selection pathway regulates the effector function of CD4 T cells
- PMID: 17724129
- PMCID: PMC2118694
- DOI: 10.1084/jem.20070321
Thymic selection pathway regulates the effector function of CD4 T cells
Abstract
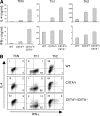
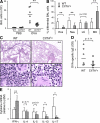
Recently, a new developmental pathway for CD4 T cells that is mediated by major histocompatibility complex class II-positive thymocytes was identified (Choi, E.Y., K.C. Jung, H.J. Park, D.H. Chung, J.S. Song, S.D. Yang, E. Simpson, and S.H. Park. 2005. Immunity. 23:387-396; Li, W., M.G. Kim, T.S. Gourley, B.P. McCarthy, D.B. Sant'angelo, and C.H. Chang. 2005. Immunity. 23:375-386). We demonstrate that thymocyte-selected CD4 (T-CD4) T cells can rapidly produce interferon gamma and interleukin (IL) 4 upon in vivo and in vitro T cell receptor stimulation. These T-CD4 T cells appear to be effector cells producing both T helper type 1 (Th1) and Th2 cytokines, and they maintain a potential to produce Th2 cytokines under Th1-skewing conditions in a signal transducer and activator of transcription 6-independent manner. The IL-4 mRNA level is high in CD4 single-positive thymocytes if they are selected on thymocytes, which is at least partly caused by enhanced histone acetylation of the IL-4 locus. However, mice that can generate T-CD4 T cells showed attenuated immune responses in an allergen-induced airway inflammation model, suggesting a protective role for T-CD4 T cells during an airway challenge. Our results imply that this thymic selection pathway plays an important role in determining the effector function of the resulting CD4 cells and in regulating immune response.
Figures





References
-
- Mosmann, T.R., H. Cherwinski, M.W. Bond, M.A. Giedlin, and R.L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 175:5–14. - PubMed
-
- Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. - PubMed
-
- Weaver, C.T., L.E. Harrington, P.R. Mangan, M. Gavrieli, and K.M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24:677–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

