MyD88-5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival
- PMID: 17724133
- PMCID: PMC2118693
- DOI: 10.1084/jem.20070868
MyD88-5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival
Abstract
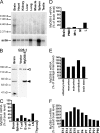
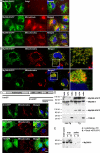
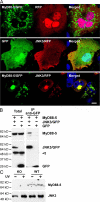
The innate immune system relies on evolutionally conserved Toll-like receptors (TLRs) to recognize diverse microbial molecular structures. Most TLRs depend on a family of adaptor proteins termed MyD88s to transduce their signals. Critical roles of MyD88-1-4 in host defense were demonstrated by defective immune responses in knockout mice. In contrast, the sites of expression and functions of vertebrate MyD88-5 have remained elusive. We show that MyD88-5 is distinct from other MyD88s in that MyD88-5 is preferentially expressed in neurons, colocalizes in part with mitochondria and JNK3, and regulates neuronal death. We prepared MyD88-5/GFP transgenic mice via a bacterial artificial chromosome to preserve its endogenous expression pattern. MyD88-5/GFP was detected chiefly in the brain, where it associated with punctate structures within neurons and copurified in part with mitochondria. In vitro, MyD88-5 co-immunoprecipitated with JNK3 and recruited JNK3 from cytosol to mitochondria. Hippocampal neurons from MyD88-5-deficient mice were protected from death after deprivation of oxygen and glucose. In contrast, MyD88-5-null macrophages behaved like wild-type cells in their response to microbial products. Thus, MyD88-5 appears unique among MyD88s in functioning to mediate stress-induced neuronal toxicity.
Figures


References
-
- Alexopoulou, L., A.C. Holt, R. Medzhitov, and R.A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 413:732–738. - PubMed
-
- Xu, Y., X. Tao, B. Shen, T. Horng, R. Medzhitov, J.L. Manley, and L. Tong. 2000. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 408:111–115. - PubMed
-
- Fitzgerald, K.A., E.M. Palsson-McDermott, A.G. Bowie, C.A. Jefferies, A.S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M.T. Harte, et al. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 413:78–83. - PubMed
-
- Horng, T., G.M. Barton, and R. Medzhitov. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835–841. - PubMed
-
- Yamamoto, M., S. Sato, H. Hemmi, H. Sanjo, S. Uematsu, T. Kaisho, K. Hoshino, O. Takeuchi, M. Kobayashi, T. Fujita, et al. 2002. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 420:324–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

