BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo
- PMID: 17724140
- PMCID: PMC1959502
- DOI: 10.2353/ajpath.2007.070168
BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo
Abstract
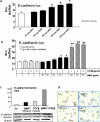
Bone morphogenic protein 7 (BMP7) counteracts physiological epithelial-to-mesenchymal transition, a process that is indicative of epithelial plasticity. Because epithelial-to-mesenchymal transition is involved in cancer, we investigated whether BMP7 plays a role in prostate cancer growth and metastasis. BMP7 expression in laser-microdissected primary human prostate cancer tissue was strongly down-regulated compared with normal prostate luminal epithelium. Furthermore, BMP7 expression in prostate cancer cell lines was inversely related to tumorigenic and metastatic potential in vivo and significantly correlated to E-cadherin/vimentin ratios. Exogenous addition of BMP7 to human prostate cancer cells dose-dependently inhibited transforming growth factor beta-induced activation of nuclear Smad3/4 complexes via ALK5 and induced E-cadherin expression. Moreover, BMP7-induced activation of nuclear Smad1/4/5 signaling transduced via BMP type I receptors was synergistically stimulated in the presence of transforming growth factor beta, a growth factor that is enriched in the bone microenvironment. Daily BMP7 administration to nude mice inhibited the growth of cancer cells in bone. In contrast, no significant growth inhibitory effect of BMP7 was observed in intraprostatic xenografts. Collectively, our observations suggest that BMP7 controls and preserves the epithelial phenotype in the human prostate and underscore a decisive role of the tumor microenvironment in mediating the therapeutic response of BMP7. Thus, BMP7 can still counteract the epithelial-to-mesenchymal transition process in the metastatic tumor, positioning BMP7 as a novel therapeutic molecule for treatment of metastatic bone disease.
Figures

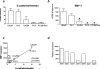



Comment in
-
BMP7: a new bone metastases prevention?Am J Pathol. 2007 Sep;171(3):739-43. doi: 10.2353/ajpath.2007.070582. Epub 2007 Aug 9. Am J Pathol. 2007. PMID: 17690188 Free PMC article. No abstract available.
Similar articles
-
Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer.Cancer Res. 2007 Sep 15;67(18):8742-51. doi: 10.1158/0008-5472.CAN-06-2490. Cancer Res. 2007. PMID: 17875715
-
Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells.Cancer Res. 2005 Jul 1;65(13):5769-77. doi: 10.1158/0008-5472.CAN-05-0289. Cancer Res. 2005. PMID: 15994952
-
BMP7: a new bone metastases prevention?Am J Pathol. 2007 Sep;171(3):739-43. doi: 10.2353/ajpath.2007.070582. Epub 2007 Aug 9. Am J Pathol. 2007. PMID: 17690188 Free PMC article. No abstract available.
-
Bone morphogenetic proteins and its receptors; therapeutic targets in cancer progression and bone metastasis?Curr Pharm Des. 2010;16(11):1291-300. doi: 10.2174/138161210791033987. Curr Pharm Des. 2010. PMID: 20166979 Review.
-
Cell death under epithelial-mesenchymal transition control in prostate cancer therapeutic response.Int J Urol. 2018 Apr;25(4):318-326. doi: 10.1111/iju.13505. Epub 2018 Jan 17. Int J Urol. 2018. PMID: 29345000 Review.
Cited by
-
To differentiate or not--routes towards metastasis.Nat Rev Cancer. 2012 May 11;12(6):425-36. doi: 10.1038/nrc3265. Nat Rev Cancer. 2012. PMID: 22576165 Review.
-
TGFβ Family Signaling Pathways in Pluripotent and Teratocarcinoma Stem Cells' Fate Decisions: Balancing Between Self-Renewal, Differentiation, and Cancer.Cells. 2019 Nov 23;8(12):1500. doi: 10.3390/cells8121500. Cells. 2019. PMID: 31771212 Free PMC article. Review.
-
Contextual effect of repression of bone morphogenetic protein activity in prostate cancer.Endocr Relat Cancer. 2013 Nov 4;20(6):861-74. doi: 10.1530/ERC-13-0100. Print 2013 Dec. Endocr Relat Cancer. 2013. PMID: 24042462 Free PMC article.
-
Targeting of α(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer.Neoplasia. 2011 Jun;13(6):516-25. doi: 10.1593/neo.11122. Neoplasia. 2011. PMID: 21677875 Free PMC article.
-
Homing of cancer cells to the bone.Cancer Microenviron. 2011 Dec;4(3):221-35. doi: 10.1007/s12307-011-0083-6. Epub 2011 Aug 9. Cancer Microenviron. 2011. PMID: 21826451 Free PMC article.
References
-
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. - PubMed
-
- Prindull G. Hypothesis: cell plasticity, linking embryonal stem cells to adult stem cell reservoirs and metastatic cancer cells? Exp Hematol. 2005;33:738–746. - PubMed
-
- Huber MA, Kraut N, Beug H. Molecular mechanisms for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. - PubMed
-
- Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-(β) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112:4557–4568. - PubMed
-
- Zavadil J, Bottinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

