Intracellular metabolism and persistence of the anti-human immunodeficiency virus activity of 2',3'-didehydro-3'-deoxy-4'-ethynylthymidine, a novel thymidine analog
- PMID: 17724147
- PMCID: PMC2151449
- DOI: 10.1128/AAC.00692-07
Intracellular metabolism and persistence of the anti-human immunodeficiency virus activity of 2',3'-didehydro-3'-deoxy-4'-ethynylthymidine, a novel thymidine analog
Abstract
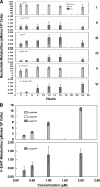
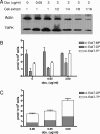
The therapeutic benefits of current antiretroviral therapy are limited by the evolution of drug-resistant virus and long-term toxicity. Novel antiretroviral compounds with activity against drug-resistant viruses are needed. 2',3'-didehydro-3'-deoxy-4'-ethynylthymidine (4'-Ed4T), a novel thymidine analog, has potent anti-human immunodeficiency virus (HIV) activity, maintains considerable activity against multidrug-resistant HIV strains, and is less inhibitory to mitochondrial DNA synthesis in cell culture than its progenitor stavudine (D4T). We investigated the intracellular metabolism and anti-HIV activity of 4'-Ed4T. The profile of 4'-Ed4T metabolites was qualitatively similar to that for zidovudine (AZT), with the monophosphate metabolite as the major metabolite, in contrast to that for D4T, with relatively poor formation of total metabolites. The first phosphorylation step for 4'-Ed4T in cells was more efficient than that for D4T but less than that for AZT. The amount of 4'-Ed4T triphosphate (4'-Ed4TTP) was higher than that of AZTTP at 24 h in culture. There was a dose-dependent accumulation of 4'-Ed4T diphosphate and 4'-Ed4TTP on up-regulation of thymidylate kinase and 3-phosphoglycerate kinase expression in Tet-On RKO cells, respectively. The anti-HIV activity of 4'-Ed4T in cells persisted even after 48 h of drug removal from culture in comparison with AZT, D4T, and nevirapine (NVP). The order of increasing persistence of anti-HIV activity of these compounds after drug removal was 4'-Ed4T > D4T > AZT > NVP. In conclusion, with the persistence of 4'-Ed4TTP and persistent anti-HIV activity in cells, we anticipate less frequent dosing and fewer patient compliance issues than for D4T. 4'-Ed4T is a promising antiviral candidate for HIV type 1 chemotherapy.
Figures



Similar articles
-
Retention of metabolites of 2',3'-didehydro-3'-deoxy-4'-ethynylthymidine, a novel anti-human immunodeficiency virus type 1 thymidine analog, in cells.Antimicrob Agents Chemother. 2009 Aug;53(8):3317-24. doi: 10.1128/AAC.00302-09. Epub 2009 May 26. Antimicrob Agents Chemother. 2009. PMID: 19470503 Free PMC article.
-
Anti-human immunodeficiency virus type 1 activity and resistance profile of 2',3'-didehydro-3'-deoxy-4'-ethynylthymidine in vitro.Antimicrob Agents Chemother. 2005 Aug;49(8):3355-60. doi: 10.1128/AAC.49.8.3355-3360.2005. Antimicrob Agents Chemother. 2005. PMID: 16048947 Free PMC article.
-
Impact of novel human immunodeficiency virus type 1 reverse transcriptase mutations P119S and T165A on 4'-ethynylthymidine analog resistance profile.Antimicrob Agents Chemother. 2009 Nov;53(11):4640-6. doi: 10.1128/AAC.00686-09. Epub 2009 Aug 24. Antimicrob Agents Chemother. 2009. PMID: 19704131 Free PMC article.
-
Stampidine as a novel nucleoside reverse transcriptase inhibit with potent anti-HIV activity.Arzneimittelforschung. 2006 Feb;56(2A):121-35. doi: 10.1055/s-0031-1296800. Arzneimittelforschung. 2006. PMID: 16570821 Review.
-
Structural requirements for efficient phosphorylation of nucleotide analogs by human thymidylate kinase.Mini Rev Med Chem. 2004 May;4(4):351-9. doi: 10.2174/1389557043403981. Mini Rev Med Chem. 2004. PMID: 15134538 Review.
Cited by
-
Function-oriented development of CXCR4 antagonists as selective human immunodeficiency virus (HIV)-1 entry inhibitors.J Med Chem. 2015 Feb 12;58(3):1452-65. doi: 10.1021/jm501772w. Epub 2015 Jan 28. J Med Chem. 2015. PMID: 25584630 Free PMC article.
-
Analysis of deoxyribonucleotide pools in human cancer cell lines using a liquid chromatography coupled with tandem mass spectrometry technique.Biochem Pharmacol. 2011 Aug 15;82(4):411-7. doi: 10.1016/j.bcp.2011.05.009. Epub 2011 May 18. Biochem Pharmacol. 2011. PMID: 21620803 Free PMC article.
-
From the chemistry of epoxy-sugar nucleosides to the discovery of anti-HIV agent 4'-ethynylstavudine-Festinavir.Curr Pharm Des. 2013;19(10):1880-97. doi: 10.2174/1381612811319100011. Curr Pharm Des. 2013. PMID: 23092278 Free PMC article. Review.
-
Comparative study of the persistence of anti-HIV activity of deoxynucleoside HIV reverse transcriptase inhibitors after removal from culture.AIDS Res Ther. 2009 Apr 22;6:5. doi: 10.1186/1742-6405-6-5. AIDS Res Ther. 2009. PMID: 19386130 Free PMC article.
-
Characterization, Dynamics, and Mechanism of CXCR4 Antagonists on a Constitutively Active Mutant.Cell Chem Biol. 2019 May 16;26(5):662-673.e7. doi: 10.1016/j.chembiol.2019.01.012. Epub 2019 Feb 28. Cell Chem Biol. 2019. PMID: 30827936 Free PMC article.
References
-
- Balzarini, J. 1994. Metabolism and mechanism of antiretroviral action of purine and pyrimidine derivatives. Pharm. World Sci. 16:113-126. - PubMed
-
- Bardsley-Elliot, A., and C. M. Perry. 2000. Nevirapine: a review of its use in the prevention and treatment of paediatric HIV infection. Paediatr. Drugs 2:373-407. - PubMed
-
- Brinkman, K., H. J. ter Hofstede, D. M. Burger, J. A. Smeitink, and P. P. Koopmans. 1998. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 12:1735-1744. - PubMed
-
- Chen, C. H., and Y. C. Cheng. 1989. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2′,3′-dideoxycytidine. J. Biol. Chem. 264:11934-11937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

