Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function
- PMID: 17724169
- PMCID: PMC2074894
- DOI: 10.1167/iovs.06-1536
Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function
Abstract
Purpose: Junctional adhesion molecules (JAMs) are a family of adhesion proteins found in intercellular junctions. Evidence suggests that JAM-A is important for the regulation of tight junction assembly and epithelial barrier function. The authors recently reported that JAM-A is expressed in rabbit corneal endothelium and that antibody to JAM-A produces corneal swelling. In the present study, they investigate JAM-A expression in the human corneal endothelium and retinal pigment epithelium (RPE) and examine the effect of a function-blocking antibody to JAM-A on the permeability of cultured RPE cell monolayers.
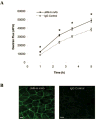
Methods: Expression of JAM-A in human corneal endothelium, human RPE tissue, and cultured ARPE-19 monolayers was assessed by immunofluorescence confocal microscopy. Localization of JAM-A was compared with the tight junction-associated protein zonula occludens-1 (ZO-1). To investigate JAM-A function in ARPE-19 cells, ARPE-19 monolayers were subjected to a calcium switch protocol to disrupt cell junctions and treated with a function-blocking antibody to JAM-A or an isotype-matched control. Dextran flux assays were performed to assess the effect of JAM-A antibody on ARPE-19 monolayer permeability.
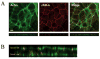
Results: Expression of JAM-A was observed in human corneal endothelium, and its distribution correlated with the tight junction-associated protein ZO-1. In addition, expression of JAM-A was observed in human RPE and in intercellular junctions of ARPE-19 monolayers. The localization pattern of JAM-A in the RPE and ARPE-19 monolayers was similar to that of ZO-1. ARPE-19 monolayers treated with antibody to JAM-A demonstrated a 33% increase in permeability to 10,000 MWt dextran compared with monolayers treated with control antibody.
Conclusions: Results of this study provide new information about JAM-A expression in tight junctions of the human corneal endothelium and human RPE. The observation that antibodies to JAM-A increase ARPE-19 monolayer permeability is consistent with previous findings of JAM-A function in epithelial tight junctions and suggests JAM-A may have a role in the regulation of RPE barrier function.
Figures





Similar articles
-
Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium.Invest Ophthalmol Vis Sci. 2009 Mar;50(3):1454-63. doi: 10.1167/iovs.08-2129. Epub 2008 Dec 5. Invest Ophthalmol Vis Sci. 2009. PMID: 19060272 Free PMC article.
-
Antibody blockade of junctional adhesion molecule-A in rabbit corneal endothelial tight junctions produces corneal swelling.Invest Ophthalmol Vis Sci. 2006 Jun;47(6):2408-16. doi: 10.1167/iovs.05-0745. Invest Ophthalmol Vis Sci. 2006. PMID: 16723450
-
Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium.J Comp Neurol. 2007 Nov 10;505(2):166-76. doi: 10.1002/cne.21489. J Comp Neurol. 2007. PMID: 17853450 Free PMC article.
-
Deletion mutants in human cytomegalovirus glycoprotein US9 are impaired in cell-cell transmission and in altering tight junctions of polarized human retinal pigment epithelial cells.Scand J Infect Dis Suppl. 1995;99:82-7. Scand J Infect Dis Suppl. 1995. PMID: 8668948 Review.
-
The JAM family of junctional adhesion molecules.Curr Opin Cell Biol. 2003 Oct;15(5):525-30. doi: 10.1016/s0955-0674(03)00104-2. Curr Opin Cell Biol. 2003. PMID: 14519386 Review.
Cited by
-
Densitometric analysis of cornea in patients with neovascular age-related macular degeneration after intravitreal aflibercept loading dose.Ther Adv Ophthalmol. 2020 Aug 27;12:2515841420950857. doi: 10.1177/2515841420950857. eCollection 2020 Jan-Dec. Ther Adv Ophthalmol. 2020. PMID: 32923942 Free PMC article.
-
Characterization and comparison of intercellular adherent junctions expressed by human corneal endothelial cells in vivo and in vitro.Invest Ophthalmol Vis Sci. 2008 Sep;49(9):3879-86. doi: 10.1167/iovs.08-1693. Epub 2008 May 23. Invest Ophthalmol Vis Sci. 2008. PMID: 18502989 Free PMC article.
-
JAM-related proteins in mucosal homeostasis and inflammation.Semin Immunopathol. 2014 Mar;36(2):211-26. doi: 10.1007/s00281-014-0421-0. Epub 2014 Mar 26. Semin Immunopathol. 2014. PMID: 24667924 Free PMC article. Review.
-
Role of JAM-A tyrosine phosphorylation in epithelial barrier dysfunction during intestinal inflammation.Mol Biol Cell. 2019 Mar 1;30(5):566-578. doi: 10.1091/mbc.E18-08-0531. Epub 2019 Jan 9. Mol Biol Cell. 2019. PMID: 30625033 Free PMC article.
-
Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium.Invest Ophthalmol Vis Sci. 2009 Mar;50(3):1454-63. doi: 10.1167/iovs.08-2129. Epub 2008 Dec 5. Invest Ophthalmol Vis Sci. 2009. PMID: 19060272 Free PMC article.
References
-
- Mandell KJ, Parkos CA. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57:857–867. - PubMed
-
- Liu Y, Nusrat A, Schnell FJ, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(pt 13):2363–2374. - PubMed
-
- Kornecki E, Walkowiak B, Naik UP, Ehrlich YH. Activation of human platelets by a stimulatory monoclonal antibody. J Biol Chem. 1990;265:10042–10048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

