Degradation of C-terminal truncated alpha A-crystallins by the ubiquitin-proteasome pathway
- PMID: 17724207
- PMCID: PMC2098745
- DOI: 10.1167/iovs.07-0196
Degradation of C-terminal truncated alpha A-crystallins by the ubiquitin-proteasome pathway
Abstract
Purpose: Calpain-mediated C-terminal cleavage of alpha A-crystallins occurs during aging and cataractogenesis. The objective of the present study was to explore the role of the ubiquitin-proteasome pathway (UPP) in degrading C-terminal truncated alpha A-crystallins.
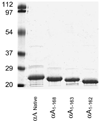
Methods: Recombinant wild-type (wt) alpha A-crystallin and C-terminal truncated alpha A(1-168)-, alpha A(1-163)-, and alpha A(1-162)-crystallins were expressed in Escherichia coli and purified to homogeneity. The wt and truncated alpha A-crystallins were labeled with (125)I, and proteolytic degradation was determined using both lens fiber lysate and reticulocyte lysate as sources of ubiquitinating and proteolytic enzymes. Far UV circular dichroism, tryptophan fluorescence intensity, and binding to the hydrophobic fluorescence probe Bis-ANS were used to characterize the wt and truncated alpha A-crystallins. Oligomer sizes of these crystallins were determined by multiangle light-scattering.
Results: Whereas wt alpha A-crystallin was degraded moderately in both lens fiber and reticulocyte lysates, alpha A(1-168)-crystallin was resistant to degradation. The susceptibility of alpha A(1-163)-crystallin to degradation was comparable to that of wt alpha A-crystallin. However, alpha A(1-162)-crystallin was much more susceptible than wt alpha A-crystallin to degradation in both lens fiber and reticulocyte lysates. The degradation of both wt and C-terminal truncated alpha A(1-162)-crystallins requires adenosine triphosphate (ATP) and was stimulated by addition of a ubiquitin-conjugating enzyme, Ubc4. The degradation was substantially inhibited by the proteasome inhibitor MG132 and a dominant negative mutant of ubiquitin, K6W-Ub, indicating that at least part of the proteolysis was mediated by the UPP. Spectroscopic analyses of wt and C-terminal truncated alpha A-crystallins revealed that C-terminal truncation of alpha A-crystallin resulted in only subtle changes in secondary structures. However, C-terminal truncations resulted in significant changes in surface hydrophobicity and thermal stability. Thus, these conformational changes may reveal or mask the signals for the ubiquitin-dependent degradation.
Conclusions: The present data demonstrate that C-terminal cleavage of alpha A-crystallin not only alters its conformation and thermal stability, but also its susceptibility to degradation by the UPP. The rapid degradation of alpha A(1-162) by the UPP may prevent its accumulation in the lens.
Figures




Similar articles
-
Glutathiolation enhances the degradation of gammaC-crystallin in lens and reticulocyte lysates, partially via the ubiquitin-proteasome pathway.Invest Ophthalmol Vis Sci. 2006 Aug;47(8):3467-73. doi: 10.1167/iovs.05-1664. Invest Ophthalmol Vis Sci. 2006. PMID: 16877417 Free PMC article.
-
Oligomerization with wt αA- and αB-crystallins reduces proteasome-mediated degradation of C-terminally truncated αA-crystallin.Invest Ophthalmol Vis Sci. 2012 May 4;53(6):2541-50. doi: 10.1167/iovs.11-9147. Invest Ophthalmol Vis Sci. 2012. PMID: 22427585 Free PMC article.
-
Ubiquitin proteasome pathway-mediated degradation of proteins: effects due to site-specific substrate deamidation.Invest Ophthalmol Vis Sci. 2010 Aug;51(8):4164-73. doi: 10.1167/iovs.09-4087. Epub 2010 Jun 30. Invest Ophthalmol Vis Sci. 2010. PMID: 20592226 Free PMC article.
-
Alpha crystallin: the quest for a homogeneous quaternary structure.Exp Eye Res. 2009 Feb;88(2):190-4. doi: 10.1016/j.exer.2008.07.007. Epub 2008 Jul 25. Exp Eye Res. 2009. PMID: 18703051 Free PMC article. Review.
-
Ubiquitin-independent proteasomal degradation during oncogenic viral infections.Biochim Biophys Acta. 2011 Dec;1816(2):147-57. doi: 10.1016/j.bbcan.2011.05.005. Epub 2011 Jun 6. Biochim Biophys Acta. 2011. PMID: 21664948 Free PMC article. Review.
Cited by
-
Structural and functional properties of NH(2)-terminal domain, core domain, and COOH-terminal extension of αA- and αB-crystallins.Mol Vis. 2011;17:2356-67. Epub 2011 Aug 31. Mol Vis. 2011. PMID: 21921988 Free PMC article.
-
Proteotoxic Stress Desensitizes TGF-beta Signaling Through Receptor Downregulation in Retinal Pigment Epithelial Cells.Curr Mol Med. 2017;17(3):189-199. doi: 10.2174/1566524017666170619113435. Curr Mol Med. 2017. PMID: 28625142 Free PMC article.
-
Glycative stress as a cause of macular degeneration.Prog Retin Eye Res. 2024 Jul;101:101260. doi: 10.1016/j.preteyeres.2024.101260. Epub 2024 Mar 21. Prog Retin Eye Res. 2024. PMID: 38521386 Free PMC article. Review.
-
Proteostasis in aging-associated ocular disease.Mol Aspects Med. 2022 Dec;88:101157. doi: 10.1016/j.mam.2022.101157. Epub 2022 Nov 29. Mol Aspects Med. 2022. PMID: 36459837 Free PMC article. Review.
-
Reversible cold-induced lens opacity in a hibernator reveals a molecular target for treating cataracts.J Clin Invest. 2024 Sep 17;134(18):e169666. doi: 10.1172/JCI169666. J Clin Invest. 2024. PMID: 39286982 Free PMC article.
References
-
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. - PubMed
-
- McDevitt DS, Hawkins JW, Jaworski CJ, Piatigorsky J. Isolation and partial characterization of the human alpha a-crystallin gene. Exp Eye Res. 1986;43:285–291. - PubMed
-
- Dubin RA, Ally AH, Chung S, Piatigorsky J. Human alpha b-crystallin gene and preferential promoter function in lens. Genomics. 1990;7:594–601. - PubMed
-
- Piatigorsky J. Molecular biology: recent studies on enzyme/crystallins and alpha-crystallin gene expression. Exp Eye Res. 1990;50:725–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

