Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord
- PMID: 17725832
- PMCID: PMC2000875
- DOI: 10.1186/1472-6793-7-9
Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord
Abstract
Background: Exogenous NGF or saline was delivered to the detrusor smooth muscle of female rats for a two-week period using osmotic mini-pumps. We then determined: (1) bladder function using conscious cystometry; (2) organization of micturition reflexes using Fos protein expression in lumbosacral (L5-S1) spinal cord neurons; (3) calcitonin gene-related peptide (CGRP)-immunoreactivity (IR) in lumbosacral spinal cord segments.
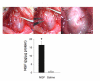
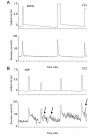
Methods: An osmotic pump infused 0.9% NaCl (n = 6) or NGF (n = 6)(2.5 microg/microl solution; 0.5 microl/hr) for two weeks into the bladder wall. NGF bladder content was determined by enzyme-linked immunoassays. Bladder function was assessed with conscious cystometry. Immunohistochemical and imaging techniques were used to determine the distribution of Fos-IR cells and CGRP expression in the L5-S1 spinal cord in saline and NGF-treated rats two hours after intravesical saline distention. Fos expression and CGRP-IR in NGF-treated rats with bladder distention was compared to that observed in cyclophosphamide (CYP; 75 mg/kg; i.p.) treated rats with bladder distention.
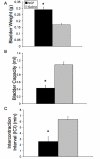
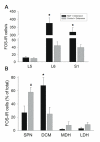
Results: Two-week infusion of NGF into the bladder wall increased bladder weight, reduced bladder capacity (60%), reduced the intercontraction interval (60%) and increased the amplitude of non-voiding contractions. NGF treatment and intravesical saline distention (2 hr) increased expression of Fos protein in L6-S1 spinal cord and altered the distribution pattern of Fos-IR cells. CGRP-IR in the lumbosacral spinal cord was also increased after NGF treatment.
Conclusion: These data suggest that NGF infusion into the bladder wall induces bladder overactivity, can reveal a "nociceptive" Fos expression pattern in the spinal cord in response to a non-noxious bladder stimulus and increases CGRP-IR in the lumbosacral spinal cord.
Figures



Similar articles
-
Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury.J Comp Neurol. 2004 Aug 2;475(4):590-603. doi: 10.1002/cne.20195. J Comp Neurol. 2004. PMID: 15236239
-
Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats.J Urol. 2002 Nov;168(5):2269-74. doi: 10.1016/S0022-5347(05)64369-8. J Urol. 2002. PMID: 12394773
-
Central inhibitory effect of intravesically applied botulinum toxin A in chronic spinal cord injury.Neurourol Urodyn. 2011 Sep;30(7):1376-81. doi: 10.1002/nau.21068. Epub 2011 Apr 20. Neurourol Urodyn. 2011. PMID: 21509809
-
[Physiopathology of overactive bladder syndrome].Urologia. 2012;79(1):24-35. doi: 10.5301/RU.2012.8972. Urologia. 2012. PMID: 22287269 Review. Italian.
-
[Menthol in the control of bladder activity: A review].Prog Urol. 2018 Sep;28(11):523-529. doi: 10.1016/j.purol.2018.07.002. Epub 2018 Aug 8. Prog Urol. 2018. PMID: 30098904 Review. French.
Cited by
-
Effects of CYP-induced cystitis on PACAP/VIP and receptor expression in micturition pathways and bladder function in mice with overexpression of NGF in urothelium.J Mol Neurosci. 2012 Nov;48(3):730-43. doi: 10.1007/s12031-012-9834-1. Epub 2012 Jun 15. J Mol Neurosci. 2012. PMID: 22700375 Free PMC article.
-
Afferent nerve regulation of bladder function in health and disease.Handb Exp Pharmacol. 2009;(194):91-138. doi: 10.1007/978-3-540-79090-7_4. Handb Exp Pharmacol. 2009. PMID: 19655106 Free PMC article. Review.
-
Bladder sensory physiology: neuroactive compounds and receptors, sensory transducers, and target-derived growth factors as targets to improve function.Am J Physiol Regul Integr Comp Physiol. 2014 Jun 15;306(12):R869-78. doi: 10.1152/ajpregu.00030.2014. Epub 2014 Apr 23. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24760999 Free PMC article. Review.
-
Mechanisms inducing autonomic dysreflexia during urinary bladder distention in rats with spinal cord injury.Spinal Cord. 2015 Mar;53(3):190-194. doi: 10.1038/sc.2014.233. Epub 2014 Dec 23. Spinal Cord. 2015. PMID: 25535154
-
Expression and function of CCL2/CCR2 in rat micturition reflexes and somatic sensitivity with urinary bladder inflammation.Am J Physiol Renal Physiol. 2013 Jul 1;305(1):F111-22. doi: 10.1152/ajprenal.00139.2013. Epub 2013 Apr 17. Am J Physiol Renal Physiol. 2013. PMID: 23594826 Free PMC article.
References
-
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

