Utility of interferon-gamma ELISPOT assay responses in highly tuberculosis-exposed patients with advanced HIV infection in South Africa
- PMID: 17725839
- PMCID: PMC2031899
- DOI: 10.1186/1471-2334-7-99
Utility of interferon-gamma ELISPOT assay responses in highly tuberculosis-exposed patients with advanced HIV infection in South Africa
Abstract
Background: Interferon-gamma (IFN-gamma) ELISPOT assays incorporating Mycobacterium tuberculosis-specific antigens are useful in the diagnosis of tuberculosis (TB) or latent infection. However, their utility in patients with advanced HIV is unknown. We studied determinants of ELISPOT responses among patients with advanced HIV infection (but without active TB) living in a South African community with very high TB notification rates.
Methods: IFN-gamma responses to ESAT-6 and CFP-10 in overnight ELISPOT assays and in 7-day whole blood assays (WBA) were compared in HIV-infected patients (HIV+, n = 40) and healthy HIV-negative controls (HIV-, n = 30) without active TB. Tuberculin skin tests (TSTs) were also done.
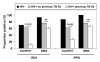
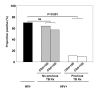
Results: ELISPOTs, WBAs and TSTs were each positive in >70% of HIV- controls, reflecting very high community exposure to M. tuberculosis. Among HIV+ patients, quantitative WBA responses and TSTs (but not the proportion of positive ELISPOT responses) were significantly impaired in those with CD4 cell counts <100 cells/mul compared to those with higher counts. In contrast, ELISPOT responses (but not WBA or TST) were strongly related to history of TB treatment; a much lower proportion of HIV+ patients who had recently completed treatment for TB (n = 19) had positive responses compared to those who had not been treated (11% versus 62%, respectively; P < 0.001). Multivariate analysis confirmed that ELISPOT responses had a strong inverse association with a history of recent TB treatment (adjusted OR = 0.06, 95%CI = 0.10-0.40, P < 0.01) and that they were independent of CD4 cell count and viral load. Among HIV+ individuals who had not received TB treatment both the magnitude and proportion of positive ELISPOT responses (but not TST or WBA) were similar to those of HIV-negative controls.
Conclusion: The proportion of positive ELISPOT responses in patients with advanced HIV infection was independent of CD4 cell count but had a strong inverse association with history of TB treatment. This concurs with the previously documented low TB risk among patients in this cohort with a history of recent treatment for TB. These data suggest ELISPOT assays may be useful for patient assessment and as an immuno-epidemiological research tool among patients with advanced HIV and warrant larger scale prospective evaluation.
Figures
Similar articles
-
Tuberculosis antigen-specific immune responses can be detected using enzyme-linked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease.Clin Exp Immunol. 2007 Nov;150(2):238-44. doi: 10.1111/j.1365-2249.2007.03477.x. Epub 2007 Aug 2. Clin Exp Immunol. 2007. PMID: 17672869 Free PMC article.
-
An observational study identifying highly tuberculosis-exposed, HIV-1-positive but persistently TB, tuberculin and IGRA negative persons with M. tuberculosis specific antibodies in Cape Town, South Africa.EBioMedicine. 2020 Nov;61:103053. doi: 10.1016/j.ebiom.2020.103053. Epub 2020 Oct 7. EBioMedicine. 2020. PMID: 33038764 Free PMC article.
-
Interferon-gamma release assays for diagnosing mycobacterium tuberculosis infection in renal dialysis patients.Clin J Am Soc Nephrol. 2008 Sep;3(5):1357-63. doi: 10.2215/CJN.01010208. Epub 2008 Jun 11. Clin J Am Soc Nephrol. 2008. PMID: 18550653 Free PMC article.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
Interferon gamma release assays: principles and practice.Enferm Infecc Microbiol Clin. 2010 Apr;28(4):245-52. doi: 10.1016/j.eimc.2009.05.012. Epub 2009 Sep 24. Enferm Infecc Microbiol Clin. 2010. PMID: 19783328 Review.
Cited by
-
Improving the diagnosis of tuberculosis: From QuantiFERON to new techniques to diagnose tuberculosis infections.Curr HIV/AIDS Rep. 2011 Sep;8(3):153-63. doi: 10.1007/s11904-011-0083-7. Curr HIV/AIDS Rep. 2011. PMID: 21660459 Review.
-
Recent advances in testing for latent TB.Chest. 2010 Dec;138(6):1456-63. doi: 10.1378/chest.10-0366. Chest. 2010. PMID: 21138881 Free PMC article. Review.
-
Epidemiology of HIV-associated tuberculosis.Curr Opin HIV AIDS. 2009 Jul;4(4):325-33. doi: 10.1097/COH.0b013e32832c7d61. Curr Opin HIV AIDS. 2009. PMID: 19532072 Free PMC article. Review.
-
Methylated HBHA produced in M. smegmatis discriminates between active and non-active tuberculosis disease among RD1-responders.PLoS One. 2011 Mar 29;6(3):e18315. doi: 10.1371/journal.pone.0018315. PLoS One. 2011. PMID: 21479248 Free PMC article.
-
A multicenter clinical evaluation of Mycobacterium tuberculosis IgG/IgM antibody detection using the colloidal gold method.Eur J Clin Microbiol Infect Dis. 2014 Nov;33(11):1989-94. doi: 10.1007/s10096-014-2150-7. Epub 2014 Jun 10. Eur J Clin Microbiol Infect Dis. 2014. PMID: 24913311 Free PMC article.
References
-
- Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. - DOI - PMC - PubMed
-
- Organisation WH. WHO declares TB an emergency in Africa.Call for "urgent and extraordinary actions" to halt a worsening epidemic.2nd September 2005 http://www.who.int/mediacentre/news/2005/africa_emergency/en/ accessed 6/9/2005. 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

