T cell activation requires mitochondrial translocation to the immunological synapse
- PMID: 17726106
- PMCID: PMC1964825
- DOI: 10.1073/pnas.0703126104
T cell activation requires mitochondrial translocation to the immunological synapse
Abstract
T helper (Th) cell activation is required for the adaptive immune response. Formation of the immunological synapse (IS) between Th cells and antigen-presenting cells is essential for Th cell activation. IS formation induces the polarization and redistribution of many signaling molecules; however, very little is known about organelle redistribution during IS formation in Th cells. We show that formation of the IS induced cytoskeleton-dependent mitochondrial redistribution to the immediate vicinity of the IS. Using total internal reflection microscopy, we found that upon stimulation, the distance between the IS and mitochondria was decreased to values<200 nm. Consequently, mitochondria close to the IS took up more Ca2+ than the ones farther away from the IS. The redistribution of mitochondria to the IS was necessary to maintain Ca2+ influx across the plasma membrane and Ca2+-dependent Th cell activation. Our results suggest that mitochondria are part of the signaling complex at the IS and that their localization close to the IS is required for Th cell activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
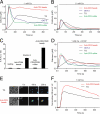

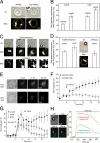
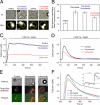

Comment in
-
Mitochondria control calcium entry at the immunological synapse.Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15171-2. doi: 10.1073/pnas.0707798104. Epub 2007 Sep 19. Proc Natl Acad Sci U S A. 2007. PMID: 17881558 Free PMC article. No abstract available.
References
-
- Friedl P, den Boer AT, Gunzer M. Nat Rev Immunol. 2005;5:532–545. - PubMed
-
- Jacobelli J, Andres PG, Boisvert J, Krummel MF. Curr Opin Immunol. 2004;16:345–352. - PubMed
-
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. Science. 1999;285:221–227. - PubMed
-
- Billadeau DD, Nolz JC, Gomez TS. Nat Rev Immunol. 2007;7:131–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

