Mitotic regulation by NIMA-related kinases
- PMID: 17727698
- PMCID: PMC2018689
- DOI: 10.1186/1747-1028-2-25
Mitotic regulation by NIMA-related kinases
Abstract
The NIMA-related kinases represent a family of serine/threonine kinases implicated in cell cycle control. The founding member of this family, the NIMA kinase of Aspergillus nidulans, as well as the fission yeast homologue Fin1, contribute to multiple aspects of mitotic progression including the timing of mitotic entry, chromatin condensation, spindle organization and cytokinesis. Mammals contain a large family of eleven NIMA-related kinases, named Nek1 to Nek11. Of these, there is now substantial evidence that Nek2, Nek6, Nek7 and Nek9 also regulate mitotic events. At least three of these kinases, as well as NIMA and Fin1, have been localized to the microtubule organizing centre of their respective species, namely the centrosome or spindle pole body. Here, they have important functions in microtubule organization and mitotic spindle assembly. Other Nek kinases have been proposed to play microtubule-dependent roles in non-dividing cells, most notably in regulating the axonemal microtubules of cilia and flagella. In this review, we discuss the evidence that NIMA-related kinases make a significant contribution to the orchestration of mitotic progression and thereby protect cells from chromosome instability. Furthermore, we highlight their potential as novel chemotherapeutic targets.
Figures
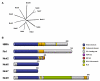
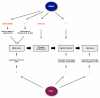
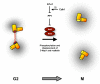

References
-
- Morris NR. Mitotic mutants of Aspergillus nidulans. Genet Res. 1975;26:237–254. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

