Pupal remodeling and the evolution and development of alternative male morphologies in horned beetles
- PMID: 17727716
- PMCID: PMC2117020
- DOI: 10.1186/1471-2148-7-151
Pupal remodeling and the evolution and development of alternative male morphologies in horned beetles
Abstract
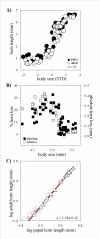
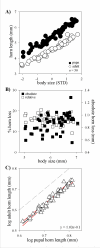
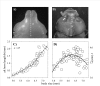
Background: How novel morphological traits originate and diversify represents a major frontier in evolutionary biology. Horned beetles are emerging as an increasingly popular model system to explore the genetic, developmental, and ecological mechanisms, as well as the interplay between them, in the genesis of novelty and diversity. The horns of beetles originate during a rapid growth phase during the prepupal stage of larval development. Differential growth during this period is either implicitly or explicitly assumed to be the sole mechanism underlying differences in horn expression within and between species. Here I focus on male horn dimorphisms, a phenomenon at the center of many studies in behavioral ecology and evolutionary development, and quantify the relative contributions of a previously ignored developmental process, pupal remodeling, to the expression of male dimorphism in three horned beetle species.
Results: Prepupal growth is not the only determinant of differences in male horn expression. Instead, following their initial prepupal growth phase, beetles may be extensively remodeled during the subsequent pupal stage in a sex and size-dependent manner. Specifically, male dimorphism in the three Onthophagus species studied here was shaped not at all, partly or entirely by such pupal remodeling rather than differential growth, suggesting that pupal remodeling is phylogenetically widespread, evolutionarily labile, and developmentally flexible.
Conclusion: This study is the first to document that male dimorphism in horned beetles is the product of two developmentaly dissociated processes: prepupal growth and pupal remodeling. More generally, adult morphology alone appears to provide few clues, if any, as to the relative contributions of both processes to the expression of alternative male morphs, underscoring the importance of developmental studies in efforts aimed at understanding the evolution of adult diversity patterns.
Figures


Similar articles
-
Pupal remodeling and the development and evolution of sexual dimorphism in horned beetles.Am Nat. 2006 Dec;168(6):711-29. doi: 10.1086/509051. Epub 2006 Oct 12. Am Nat. 2006. PMID: 17109315
-
Diverse developmental mechanisms contribute to different levels of diversity in horned beetles.Evol Dev. 2005 May-Jun;7(3):175-85. doi: 10.1111/j.1525-142X.2005.05020.x. Evol Dev. 2005. PMID: 15876190
-
When ontogeny reveals what phylogeny hides: gain and loss of horns during development and evolution of horned beetles.Evolution. 2006 Nov;60(11):2329-41. Evolution. 2006. PMID: 17236424
-
Beetle horns and horned beetles: emerging models in developmental evolution and ecology.Wiley Interdiscip Rev Dev Biol. 2013 May-Jun;2(3):405-18. doi: 10.1002/wdev.81. Epub 2012 Jul 30. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 23799584 Free PMC article. Review.
-
Developmental regulation and evolution of scaling: novel insights through the study of Onthophagus beetles.Curr Opin Insect Sci. 2017 Feb;19:52-60. doi: 10.1016/j.cois.2016.11.004. Epub 2016 Dec 5. Curr Opin Insect Sci. 2017. PMID: 28521943 Review.
Cited by
-
Developmental decoupling of alternative phenotypes: insights from the transcriptomes of horn-polyphenic beetles.Evolution. 2011 Jan;65(1):231-45. doi: 10.1111/j.1558-5646.2010.01106.x. Epub 2010 Sep 24. Evolution. 2011. PMID: 20731717 Free PMC article.
-
Diversification of doublesex function underlies morph-, sex-, and species-specific development of beetle horns.Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20526-31. doi: 10.1073/pnas.1118589109. Epub 2012 Nov 26. Proc Natl Acad Sci U S A. 2012. PMID: 23184999 Free PMC article.
-
Epigenetic mechanisms underlying developmental plasticity in horned beetles.Genet Res Int. 2012;2012:576303. doi: 10.1155/2012/576303. Epub 2012 Mar 5. Genet Res Int. 2012. PMID: 22567393 Free PMC article.
-
Integrating evolutionarily novel horns within the deeply conserved insect head.BMC Biol. 2020 Apr 20;18(1):41. doi: 10.1186/s12915-020-00773-9. BMC Biol. 2020. PMID: 32312271 Free PMC article.
-
Differential recruitment of limb patterning genes during development and diversification of beetle horns.Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):8992-7. doi: 10.1073/pnas.0809668106. Epub 2009 May 18. Proc Natl Acad Sci U S A. 2009. PMID: 19451631 Free PMC article.
References
-
- Thornhill R, Moller AP. The relative importance of size and asymmetry in sexual selection. Behav Ecol. 1998;9:546–551. doi: 10.1093/beheco/9.6.546. - DOI
-
- Klingenberg CP, Nijhout HF. Competition among growing organs and developmental control of morphological asymmetry. Proc Roy Soc London B. 1998;265:1135–1139. doi: 10.1098/rspb.1998.0409. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

