Targeting APOBEC3A to the viral nucleoprotein complex confers antiviral activity
- PMID: 17727729
- PMCID: PMC2018723
- DOI: 10.1186/1742-4690-4-61
Targeting APOBEC3A to the viral nucleoprotein complex confers antiviral activity
Abstract
Background: APOBEC3 (A3) proteins constitute a family of cytidine deaminases that provide intracellular resistance to retrovirus replication and to transposition of endogenous retroelements. A3A has significant homology to the C-terminus of A3G but has only a single cytidine deaminase active site (CDA), unlike A3G, which has a second N-terminal CDA previously found to be important for Vif sensitivity and virus encapsidation. A3A is packaged into HIV-1 virions but, unlike A3G, does not have antiviral properties. Here, we investigated the reason for the lack of A3A antiviral activity.
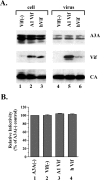
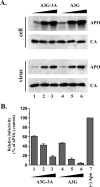
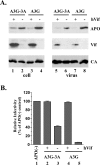
Results: Sequence alignment of A3G and A3A revealed significant homology of A3A to the C-terminal region of A3G. However, while A3G co-purified with detergent-resistant viral nucleoprotein complexes (NPC), virus-associated A3A was highly detergent-sensitive leading us to speculate that the ability to assemble into NPC may be a property conveyed by the A3G N-terminus. To test this model, we constructed an A3G-3A chimeric protein, in which the N-terminal half of A3G was fused to A3A. Interestingly, the A3G-3A chimera was packaged into HIV-1 particles and, unlike A3A, associated with the viral NPC. Furthermore, the A3G-3A chimera displayed strong antiviral activity against HIV-1 and was sensitive to inhibition by HIV-1 Vif.
Conclusion: Our results suggest that the A3G N-terminal domain carries determinants important for targeting the protein to viral NPCs. Transfer of this domain to A3A results in A3A targeting to viral NPCs and confers antiviral activity.
Figures


Similar articles
-
APOBEC3 degradation is the primary function of HIV-1 Vif determining virion infectivity in the myeloid cell line THP-1.mBio. 2023 Aug 31;14(4):e0078223. doi: 10.1128/mbio.00782-23. Epub 2023 Aug 9. mBio. 2023. PMID: 37555667 Free PMC article.
-
The activity spectrum of Vif from multiple HIV-1 subtypes against APOBEC3G, APOBEC3F, and APOBEC3H.J Virol. 2012 Jan;86(1):49-59. doi: 10.1128/JVI.06082-11. Epub 2011 Oct 19. J Virol. 2012. PMID: 22013041 Free PMC article.
-
Vpr.A3A chimera inhibits HIV replication.J Biol Chem. 2008 Feb 1;283(5):2518-25. doi: 10.1074/jbc.M706436200. Epub 2007 Dec 5. J Biol Chem. 2008. PMID: 18057006
-
APOBEC deaminases as cellular antiviral factors: a novel natural host defense mechanism.Med Sci Monit. 2006 May;12(5):RA92-8. Med Sci Monit. 2006. PMID: 16641889 Review.
-
Lentiviral Vif: viral hijacker of the ubiquitin-proteasome system.Int J Hematol. 2006 Apr;83(3):208-12. doi: 10.1532/IJH97.06013. Int J Hematol. 2006. PMID: 16720549 Review.
Cited by
-
Association of potent human antiviral cytidine deaminases with 7SL RNA and viral RNP in HIV-1 virions.J Virol. 2010 Dec;84(24):12903-13. doi: 10.1128/JVI.01632-10. Epub 2010 Oct 6. J Virol. 2010. PMID: 20926562 Free PMC article.
-
APOBEC3G encapsidation into HIV-1 virions: which RNA is it?Retrovirology. 2008 Jul 2;5:55. doi: 10.1186/1742-4690-5-55. Retrovirology. 2008. PMID: 18597677 Free PMC article.
-
APOBEC3 degradation is the primary function of HIV-1 Vif determining virion infectivity in the myeloid cell line THP-1.mBio. 2023 Aug 31;14(4):e0078223. doi: 10.1128/mbio.00782-23. Epub 2023 Aug 9. mBio. 2023. PMID: 37555667 Free PMC article.
-
APOBEC3 degradation is the primary function of HIV-1 Vif for virus replication in the myeloid cell line THP-1.bioRxiv [Preprint]. 2023 Mar 29:2023.03.28.534666. doi: 10.1101/2023.03.28.534666. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0078223. doi: 10.1128/mbio.00782-23. PMID: 37034786 Free PMC article. Updated. Preprint.
-
An overview of the functions and mechanisms of APOBEC3A in tumorigenesis.Acta Pharm Sin B. 2024 Nov;14(11):4637-4648. doi: 10.1016/j.apsb.2024.08.020. Epub 2024 Aug 27. Acta Pharm Sin B. 2024. PMID: 39664421 Free PMC article. Review.
References
-
- Kao S, Khan MA, Miyagi E, Plishka R, Buckler-White A, Strebel K. The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity. J Virol. 2003;77:11398–11407. doi: 10.1128/JVI.77.21.11398-11407.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

