TGF-beta mediated Msx2 expression controls occipital somites-derived caudal region of skull development
- PMID: 17727833
- PMCID: PMC3337706
- DOI: 10.1016/j.ydbio.2007.07.038
TGF-beta mediated Msx2 expression controls occipital somites-derived caudal region of skull development
Abstract
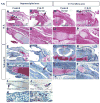
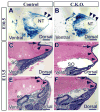
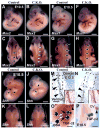
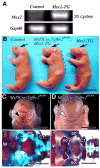
Craniofacial development involves cranial neural crest (CNC) and mesoderm-derived cells. TGF-beta signaling plays a critical role in instructing CNC cells to form the craniofacial skeleton. However, it is not known how TGF-beta signaling regulates the fate of mesoderm-derived cells during craniofacial development. In this study, we show that occipital somites contribute to the caudal region of mammalian skull development. Conditional inactivation of Tgfbr2 in mesoderm-derived cells results in defects of the supraoccipital bone with meningoencephalocele and discontinuity of the neural arch of the C1 vertebra. At the cellular level, loss of TGF-beta signaling causes decreased chondrocyte proliferation and premature differentiation of cartilage to bone. Expression of Msx2, a critical factor in the formation of the dorsoventral axis, is diminished in the Tgfbr2 mutant. Significantly, overexpression of Msx2 in Myf5-Cre;Tgfbr2flox/flox mice partially rescues supraoccipital bone development. These results suggest that the TGF-beta/Msx2 signaling cascade is critical for development of the caudal region of the skull.
Figures





Similar articles
-
Noncanonical transforming growth factor β (TGFβ) signaling in cranial neural crest cells causes tongue muscle developmental defects.J Biol Chem. 2013 Oct 11;288(41):29760-70. doi: 10.1074/jbc.M113.493551. Epub 2013 Aug 15. J Biol Chem. 2013. PMID: 23950180 Free PMC article.
-
Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects.Development. 2003 Nov;130(21):5269-80. doi: 10.1242/dev.00708. Development. 2003. PMID: 12975342
-
The role of TGF-beta signaling in regulating chondrogenesis and osteogenesis during mandibular development.Dev Biol. 2007 Mar 1;303(1):391-404. doi: 10.1016/j.ydbio.2006.11.025. Epub 2006 Nov 21. Dev Biol. 2007. PMID: 17204263 Free PMC article.
-
TGF-beta signaling and its functional significance in regulating the fate of cranial neural crest cells.Crit Rev Oral Biol Med. 2003;14(2):78-88. doi: 10.1177/154411130301400202. Crit Rev Oral Biol Med. 2003. PMID: 12764071 Review.
-
Contribution of cranial neural crest cells to mouse skull development.Int J Dev Biol. 2017;61(8-9):495-503. doi: 10.1387/ijdb.170051gc. Int J Dev Biol. 2017. PMID: 29139535 Review.
Cited by
-
Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins.Stem Cells. 2010 Nov;28(11):2027-40. doi: 10.1002/stem.525. Stem Cells. 2010. PMID: 20848654 Free PMC article.
-
Periocular neural crest cell differentiation into corneal endothelium is influenced by signals in the nascent corneal environment.Dev Biol. 2020 Sep 15;465(2):119-129. doi: 10.1016/j.ydbio.2020.06.012. Epub 2020 Jul 19. Dev Biol. 2020. PMID: 32697973 Free PMC article.
-
MSX2 suppression through inhibition of TGFβ signaling enhances hematopoietic differentiation of human embryonic stem cells.Stem Cell Res Ther. 2020 Apr 5;11(1):147. doi: 10.1186/s13287-020-01653-3. Stem Cell Res Ther. 2020. PMID: 32248833 Free PMC article.
-
Initiation of early osteoblast differentiation events through the direct transcriptional regulation of Msx2 by FOXC1.PLoS One. 2012;7(11):e49095. doi: 10.1371/journal.pone.0049095. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23145080 Free PMC article.
-
The human occipital bone: review and update on its embryology and molecular development.Childs Nerv Syst. 2015 Dec;31(12):2217-23. doi: 10.1007/s00381-015-2870-8. Epub 2015 Aug 18. Childs Nerv Syst. 2015. PMID: 26280629 Review.
References
-
- Alappat S, Zhang ZY, Chen YP. Msx homeobox gene family and craniofacial development. Cell Res. 2003;13:429–442. - PubMed
-
- Alvarez J, Horton J, Sohn P, Serra R. The perichondrium plays an important role in mediating the effects of TGF-beta1 on endochondral bone formation. Dev Dyn. 2001;221:311–321. - PubMed
-
- Alvarez J, Sohn P, Zeng X, Doetschman T, Robbins DJ, Serra R. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–1924. - PubMed
-
- Bach A, Lallemand Y, Nicola MA, Ramos C, Mathis L, Maufras M, Robert B. Msx1 is required for dorsal diencephalon patterning. Development. 2003;130:4025–4036. - PubMed
-
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

