Evolution of human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitopes: fitness-balanced escape
- PMID: 17728222
- PMCID: PMC2169017
- DOI: 10.1128/JVI.01277-07
Evolution of human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitopes: fitness-balanced escape
Abstract
CD8(+) cytotoxic T lymphocytes (CTL) are strong mediators of human immunodeficiency virus type 1 (HIV-1) control, yet HIV-1 frequently mutates to escape CTL recognition. In an analysis of sequences in the Los Alamos HIV-1 database, we show that emerging CTL escape mutations were more often present at lower frequencies than the amino acid(s) that they replaced. Furthermore, epitopes that underwent escape contained amino acid sites of high variability, whereas epitopes persisting at high frequencies lacked highly variable sites. We therefore infer that escape mutations are likely to be associated with weak functional constraints on the viral protein. This was supported by an extensive analysis of one subject for whom all escape mutations within defined CTL epitopes were studied and by an analysis of all reported escape mutations of defined CTL epitopes in the HIV Immunology Database. In one of these defined epitopes, escape mutations involving the substitution of amino acids with lower database frequencies occurred, and the epitope soon reverted back to the sensitive form. We further show that this escape mutation substantially diminished viral fitness in in vitro competition assays. Coincident with the reversion in vivo, we observed the fixation of a mutation 3 amino acids C terminal to the epitope, coincident with the ablation of the corresponding CTL response. The C-terminal mutation did not restore replication fitness reduced by the escape mutation in the epitope and by itself had little effect on replication fitness. Therefore, this C-terminal mutation presumably impaired the processing and presentation of the epitope. Finally, for one persistent epitope, CTL cross-reactivity to a mutant form may have suppressed the mutant to undetected levels, whereas for two other persistent epitopes, each of two mutants showed poor cross-reactivity and appeared in the subject at later time points. Thus, a viral dynamic exists between the advantage of immune escape, peptide cross-reactivity, and the disadvantage of lost replication fitness, with the balance playing an important role in determining whether a CTL epitope will persist or decline during infection.
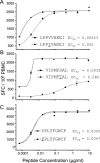
Figures



Similar articles
-
Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response.PLoS Pathog. 2009 Apr;5(4):e1000365. doi: 10.1371/journal.ppat.1000365. Epub 2009 Apr 3. PLoS Pathog. 2009. PMID: 19343217 Free PMC article.
-
Fitness-Balanced Escape Determines Resolution of Dynamic Founder Virus Escape Processes in HIV-1 Infection.J Virol. 2015 Oct;89(20):10303-18. doi: 10.1128/JVI.01876-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223634 Free PMC article.
-
Molecular and functional analysis of a conserved CTL epitope in HIV-1 p24 recognized from a long-term nonprogressor: constraints on immune escape associated with targeting a sequence essential for viral replication.J Immunol. 1999 Mar 15;162(6):3727-34. J Immunol. 1999. PMID: 10092836
-
Escape of human immunodeficiency virus from immune control.Annu Rev Immunol. 1997;15:271-96. doi: 10.1146/annurev.immunol.15.1.271. Annu Rev Immunol. 1997. PMID: 9143689 Review.
-
Co-evolution of human immunodeficiency virus and cytotoxic T-lymphocyte responses.Immunol Rev. 1997 Oct;159:17-29. doi: 10.1111/j.1600-065x.1997.tb01004.x. Immunol Rev. 1997. PMID: 9416500 Review.
Cited by
-
Preclinical Assessment of Mutant Human TRIM5α as an Anti-HIV-1 Transgene.Hum Gene Ther. 2015 Oct;26(10):664-79. doi: 10.1089/hum.2015.059. Epub 2015 Aug 6. Hum Gene Ther. 2015. PMID: 26076730 Free PMC article.
-
Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response.PLoS Pathog. 2009 Apr;5(4):e1000365. doi: 10.1371/journal.ppat.1000365. Epub 2009 Apr 3. PLoS Pathog. 2009. PMID: 19343217 Free PMC article.
-
Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads.PLoS One. 2008 Jan 9;3(1):e1424. doi: 10.1371/journal.pone.0001424. PLoS One. 2008. PMID: 18183304 Free PMC article.
-
Conserved HIV-1 epitopes continuously elicit subdominant cytotoxic T-lymphocyte responses.J Infect Dis. 2009 Dec 15;200(12):1825-33. doi: 10.1086/648401. J Infect Dis. 2009. PMID: 19909083 Free PMC article.
-
Novel cytotoxic T-lymphocyte escape mutation by a three-amino-acid insertion in the human immunodeficiency virus type 1 p6Pol and p6Gag late domain associated with drug resistance.J Virol. 2008 Jan;82(1):495-502. doi: 10.1128/JVI.01096-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942528 Free PMC article.
References
-
- Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. R. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. - PMC - PubMed
-
- Alexander-Miller, M. A., K. C. Parker, T. Tsukui, C. D. Pendleton, J. E. Coligan, and J. A. Berzofsky. 1996. Molecular analysis of presentation by HLA-A2.1 of a promiscuously binding V3 loop peptide from the HIV-envelope protein to human cytotoxic T lymphocytes. Int. Immunol. 8:641-649. - PubMed
-
- Allen, T. M., M. Altfeld, S. C. Geer, E. T. Kalife, C. Moore, K. M. O'Sullivan, I. DeSouza, M. E. Feeney, R. L. Eldridge, E. L. Maier, D. E. Kaufmann, M. P. Lahaie, L. Reyor, G. Tanzi, M. N. Johnston, C. Brander, R. Draenert, J. K. Rockstroh, H. Jessen, E. S. Rosenberg, S. A. Mallal, and B. D. Walker. 2005. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J. Virol. 79:13239-13249. - PMC - PubMed
-
- Allen, T. M., M. Altfeld, X. G. Yu, K. M. O'Sullivan, M. Lichterfeld, S. Le Gall, M. John, B. R. Mothe, P. K. Lee, E. T. Kalife, D. E. Cohen, K. A. Freedberg, D. A. Strick, M. N. Johnston, A. Sette, E. S. Rosenberg, S. A. Mallal, P. J. R. Goulder, C. Brander, and B. D. Walker. 2004. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J. Virol. 78:7069-7078. - PMC - PubMed
-
- Allen, T. M., X. G. Yu, E. T. Kalife, L. L. Reyor, M. Lichterfeld, M. John, M. Cheng, R. L. Allgaier, S. Mui, N. Frahm, G. Alter, N. V. Brown, M. N. Johnston, E. S. Rosenberg, S. A. Mallal, C. Brander, B. D. Walker, and M. Altfeld. 2005. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J. Virol. 79:12952-12960. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

