Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses
- PMID: 17728240
- PMCID: PMC2169007
- DOI: 10.1128/JVI.00381-07
Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses
Abstract
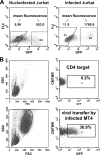
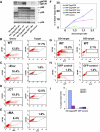
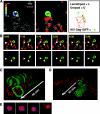
Cell-free human immunodeficiency virus type 1 (HIV-1) can initiate infections, but contact between infected and uninfected T cells can enhance viral spread through intercellular structures called virological synapses (VS). The relative contribution of VS to cell-free viral transfer has not been carefully measured. Using an ultrasensitive, fluorescent virus transfer assay, we estimate that when VS between HIV-expressing Jurkat T cells and primary CD4(+) T cells are formed, cell-associated transfer of virus is 18,000-fold more efficient than uptake of cell-free virus. Furthermore, in contrast to cell-free virus uptake, the VS deposits virus rapidly into focal, trypsin-resistant compartments in target T cells. This massive virus internalization requires Env-CD4 receptor interactions but is resistant to inhibition by patient-derived neutralizing antisera that inhibit homologous cell-free virus. Deleting the Env cytoplasmic tail does not abrogate VS-mediated transfer, but it renders the VS sensitive to neutralizing antibodies, suggesting that the tail limits exposure of VS-neutralizing epitopes on the surface of infected cells. Dynamic live imaging of the VS reveals that HIV-expressing cells are polarized and make sustained, Env-dependent contacts with target cells through uropod-like structures. The polarized T-cell morphology, Env-CD4 coordinated adhesion, and viral transfer from HIV-infected to uninfected cells suggest that VS allows HIV-1 to evade antibody neutralization and to disseminate efficiently. Future studies will discern to what extent this massive viral transfer contributes to productive infection or viral dissemination through the migration of virus-carrying T cells.
Figures

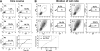

References
-
- Blanco, J., B. Bosch, M. T. Fernandez-Figueras, J. Barretina, B. Clotet, and J. A. Este. 2004. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J. Biol. Chem. 279:51305-51314. - PubMed
-
- Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 3:42-47. - PubMed
-
- Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

