Versatile applications of transcriptional pulsing to study mRNA turnover in mammalian cells
- PMID: 17728382
- PMCID: PMC1986818
- DOI: 10.1261/rna.663507
Versatile applications of transcriptional pulsing to study mRNA turnover in mammalian cells
Abstract
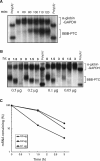
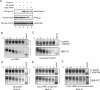
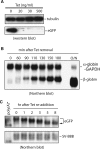
Development of transcriptional pulsing approaches using the c-fos and Tet-off promoter systems greatly facilitated studies of mRNA turnover in mammalian cells. However, optimal protocols for these approaches vary for different cell types and/or physiological conditions, limiting their widespread application. In this study, we have further optimized transcriptional pulsing systems for different cell lines and developed new protocols to facilitate investigation of various aspects of mRNA turnover. We apply the Tet-off transcriptional pulsing strategy to investigate ARE-mediated mRNA decay in human erythroleukemic K562 cells arrested at various phases of the cell cycle by pharmacological inhibitors. This application facilitates studies of the role of mRNA stability in control of cell-cycle dependent gene expression. To advance the investigation of factors involved in mRNA turnover and its regulation, we have also incorporated recently developed transfection and siRNA reagents into the transcriptional pulsing approach. Using these protocols, siRNA and DNA plasmids can be effectively cotransfected into mouse NIH3T3 cells to obtain high knockdown efficiency. Moreover, we have established a tTA-harboring stable line using human bronchial epithelial BEAS-2B cells and applied the transcriptional pulsing approach to monitor mRNA deadenylation and decay kinetics in this cell system. This broadens the application of the transcriptional pulsing system to investigate the regulation of mRNA turnover related to allergic inflammation. Critical factors that need to be considered when employing these approaches are characterized and discussed.
Figures



Similar articles
-
Messenger RNA half-life measurements in mammalian cells.Methods Enzymol. 2008;448:335-57. doi: 10.1016/S0076-6879(08)02617-7. Methods Enzymol. 2008. PMID: 19111184 Free PMC article.
-
A broader role for AU-rich element-mediated mRNA turnover revealed by a new transcriptional pulse strategy.Nucleic Acids Res. 1998 Jan 15;26(2):558-65. doi: 10.1093/nar/26.2.558. Nucleic Acids Res. 1998. PMID: 9421516 Free PMC article.
-
Transcriptional pulsing approaches for analysis of mRNA turnover in mammalian cells.Methods. 1999 Jan;17(1):11-20. doi: 10.1006/meth.1998.0702. Methods. 1999. PMID: 10075878
-
Antisense targeting of c-fos transcripts inhibits serum- and TGF-beta 1-stimulated PAI-1 gene expression and directed motility in renal epithelial cells.Cell Motil Cytoskeleton. 2001 Mar;48(3):163-74. doi: 10.1002/1097-0169(200103)48:3<163::AID-CM1006>3.0.CO;2-C. Cell Motil Cytoskeleton. 2001. PMID: 11223948
-
Discontinuous transcription.Nucleus. 2018 Jan 1;9(1):149-160. doi: 10.1080/19491034.2017.1419112. Nucleus. 2018. PMID: 29285985 Free PMC article. Review.
Cited by
-
Chapter 4. Evaluating the control of mRNA decay in fission yeast.Methods Enzymol. 2008;449:73-95. doi: 10.1016/S0076-6879(08)02404-X. Methods Enzymol. 2008. PMID: 19215754 Free PMC article.
-
RNA Foci, CUGBP1, and ZNF9 are the primary targets of the mutant CUG and CCUG repeats expanded in myotonic dystrophies type 1 and type 2.Am J Pathol. 2011 Nov;179(5):2475-89. doi: 10.1016/j.ajpath.2011.07.013. Epub 2011 Sep 1. Am J Pathol. 2011. PMID: 21889481 Free PMC article.
-
LARP1 and LARP4: up close with PABP for mRNA 3' poly(A) protection and stabilization.RNA Biol. 2021 Feb;18(2):259-274. doi: 10.1080/15476286.2020.1868753. Epub 2021 Jan 31. RNA Biol. 2021. PMID: 33522422 Free PMC article. Review.
-
Massively parallel functional annotation of 3' untranslated regions.Nat Biotechnol. 2014 Apr;32(4):387-91. doi: 10.1038/nbt.2851. Epub 2014 Mar 16. Nat Biotechnol. 2014. PMID: 24633241 Free PMC article.
-
Messenger RNA half-life measurements in mammalian cells.Methods Enzymol. 2008;448:335-57. doi: 10.1016/S0076-6879(08)02617-7. Methods Enzymol. 2008. PMID: 19111184 Free PMC article.
References
-
- Atasoy, U., Curry, S.L., Lopez de Silanes, I., Shyu, A.-B., Casolaro, V., Gorospe, M., Stellato, C. Regulation of eotaxin gene expression by TNF-α and IL-4 through mRNA stabilization: Involvement of the RNA-binding protein HuR. J. Immunol. 2003;171:4369–4378. - PubMed
-
- Atsuta, J., Sterbinsky, S.A., Plitt, J., Schwiebert, L.M., Bochner, B.S., Schleimer, R.P. Phenotyping and cytokine regulation of the BEAS-2B human bronchial epithelial cell: Demonstration of inducible expression of the adhesion molecules VCAM-1 and ICAM-1. Am. J. Respir. Cell Mol. Biol. 1997;17:571–582. - PubMed
-
- Belasco, J.G., Greenberg, M.E. Control of messenger RNA stability. In: Belasco J.G., Brawerman G., editors. Control of messenger RNA stability. Academic Press; San Diego: 1993. pp. 199–210.
-
- Chang, T.-C., Yamashita, A., Chen, C.-Y.A., Yamashita, Y., Zhu, W., Durdan, S., Kahvejian, A., Sonenberg, N., Shyu, A.-B. UNR, a new partner of poly(A)-binding protein, plays a key role in translationally coupled mRNA turnover mediated by the c-fos major coding-region determinant. Genes & Dev. 2004;18:2010–2023. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
