Amyloid beta protein modulates glutamate-mediated neurotransmission in the rat basal forebrain: involvement of presynaptic neuronal nicotinic acetylcholine and metabotropic glutamate receptors
- PMID: 17728440
- PMCID: PMC6673123
- DOI: 10.1523/JNEUROSCI.1843-07.2007
Amyloid beta protein modulates glutamate-mediated neurotransmission in the rat basal forebrain: involvement of presynaptic neuronal nicotinic acetylcholine and metabotropic glutamate receptors
Abstract
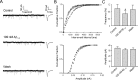
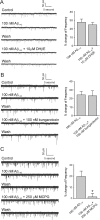
Amyloid beta (Abeta) protein, a 39-43 amino acid peptide deposited in brains of individuals with Alzheimer's disease (AD), has been shown to interact directly with a number of receptor targets including neuronal nicotinic acetylcholine receptors (nAChRs) and glutamate receptors. In this study, we investigated the synaptic effects of Abeta(1-42) on glutamate-mediated neurotransmission in the diagonal band of Broca (DBB), a cholinergic basal forebrain nucleus. Glutamatergic miniature EPSCs (mEPSCs) were recorded using whole-cell patch-clamp recordings from identified cholinergic DBB neurons in rat forebrain slices. In 54% of DBB neurons, bath application of Abeta(1-42) (100 nM), but not Abeta(42-1) (inverse fragment), significantly increased the frequency of mEPSCs without affecting amplitude or kinetic parameters (rise or decay time). In 32% of DBB neurons, bath application of Abeta(1-42) significantly decreased only the frequency but not amplitude of mEPSCs. Application of dihydro-beta-erythroidine (DHbetaE) (an antagonist for the alpha4beta2 subtype of nAChRs) but not alpha-bungarotoxin (an antagonist for the alpha7 subtype of nAChRs) blocked Abeta(1-42)-mediated increases in mEPSC frequency. The Abeta(1-42)-mediated increase in glutamatergic transmission is thus presynaptic and mediated via non-alpha7 AChRs. In contrast, Abeta(1-42)-mediated decreases in mEPSC frequency could not be antagonized by either DHbetaE or alpha-bungarotoxin. However, the Abeta(1-42)-evoked depression in mEPSC frequency was antagonized by (RS)-alpha-methyl-4-carboxyphenyglycine, a nonselective group I/II metabotropic glutamate receptor antagonist. These observations provide further insight into the mechanisms whereby Abeta affects synaptic function in the brain and may be relevant in the context of synaptic failure observed in AD.
Figures



References
-
- Alkondon M, Rocha ES, Maelicke A, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat brain. α-Bungarotoxin-sensitive nicotinic receptors in olfactory bulb neurons and presynaptic modulation of glutamate release. J Pharmacol Exp Ther. 1996;278:1460–1471. - PubMed
-
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. - PubMed
-
- Ashenafi S, Fuente A, Criado JM, Riolobos AS, Heredia M, Yajeya J. Beta-amyloid peptides25–35 depresses excitatory synaptic transmission in the rat basolateral amygdala “in vitro”. Neurobiol Aging. 2005;26:419–428. - PubMed
-
- Blanchard BJ, Thomas VL, Ingram VM. Related mechanism of membrane depolarization caused by the Alzheimer Abeta1–42 peptide. Biochem Biophys Res Commun. 2002;293:1197–1203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
