Differential abilities of SNAP-25 homologs to support neuronal function
- PMID: 17728451
- PMCID: PMC6673127
- DOI: 10.1523/JNEUROSCI.5092-06.2007
Differential abilities of SNAP-25 homologs to support neuronal function
Abstract
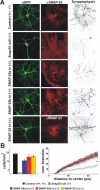
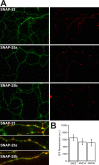
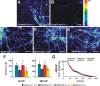
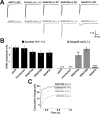
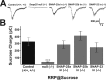
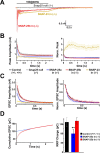
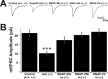
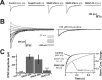
The SNAP receptor (SNARE) complex, consisting of synaptosome-associated protein of 25 kDa (SNAP-25), synaptobrevin-2, and syntaxin-1, is involved in synaptic vesicles exocytosis. In addition, SNAP-25 has been implicated in constitutive exocytosis processes required for neurite outgrowth. However, at least three isoforms of SNAP-25 have been reported from neurons: SNAP-23, which is also present in non-neuronal cells, and the two alternative splice variants SNAP-25a and SNAP-25b. Here, we studied the differential ability of these isoforms to support the functions previously broadly ascribed to "SNAP-25." We studied the rescue of snap-25 null neurons in culture with different SNAP-25 homologs. We find that deletion of SNAP-25 leads to strongly reduced neuron survival, and, in the few surviving cells, impaired arborization, reduced spontaneous release, and complete arrest of evoked release. Lentiviral expression of SNAP-25a, SNAP-25b, or SNAP-23 rescued neuronal survival, arborization, amplitude, and frequency of spontaneous events. Also evoked release was rescued by all isoforms, but synchronous release required SNAP-25a/b in both glutamatergic and GABAergic neurons. SNAP-23 supported asynchronous release only, reminiscent of synaptotagmin-1 null neurons. SNAP-25b was superior to SNAP-25a in vesicle priming, resembling the shift to larger releasable vesicle pools that accompanies synaptic maturation. These data demonstrate a differential ability of SNAP-25b, SNAP-25a, and SNAP-23 to support neuronal function.
Figures
References
-
- Bark IC. Structure of the chicken gene for SNAP-25 reveals duplicated exon encoding distinct isoforms of the protein. J Mol Biol. 1993;233:67–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
