Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU
- PMID: 17728714
- PMCID: PMC2633872
- DOI: 10.1038/nature06115
Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU
Abstract
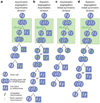
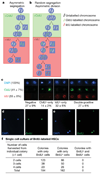
Stem cells are proposed to segregate chromosomes asymmetrically during self-renewing divisions so that older ('immortal') DNA strands are retained in daughter stem cells whereas newly synthesized strands segregate to differentiating cells. Stem cells are also proposed to retain DNA labels, such as 5-bromo-2-deoxyuridine (BrdU), either because they segregate chromosomes asymmetrically or because they divide slowly. However, the purity of stem cells among BrdU-label-retaining cells has not been documented in any tissue, and the 'immortal strand hypothesis' has not been tested in a system with definitive stem cell markers. Here we tested these hypotheses in haematopoietic stem cells (HSCs), which can be highly purified using well characterized markers. We administered BrdU to newborn mice, mice treated with cyclophosphamide and granulocyte colony-stimulating factor, and normal adult mice for 4 to 10 days, followed by 70 days without BrdU. In each case, less than 6% of HSCs retained BrdU and less than 0.5% of all BrdU-retaining haematopoietic cells were HSCs, revealing that BrdU has poor specificity and poor sensitivity as an HSC marker. Sequential administration of 5-chloro-2-deoxyuridine and 5-iodo-2-deoxyuridine indicated that all HSCs segregate their chromosomes randomly. Division of individual HSCs in culture revealed no asymmetric segregation of the label. Thus, HSCs cannot be identified on the basis of BrdU-label retention and do not retain older DNA strands during division, indicating that these are not general properties of stem cells.
Figures


References
-
- Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. - PubMed
-
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. - PubMed
-
- Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–687. - PubMed
-
- Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nature Cell Biol. 2006;8:677–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

