Molecular mechanisms involved in intestinal iron absorption
- PMID: 17729393
- PMCID: PMC4611193
- DOI: 10.3748/wjg.v13.i35.4716
Molecular mechanisms involved in intestinal iron absorption
Abstract
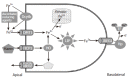
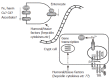
Iron is an essential trace metal in the human diet due to its obligate role in a number of metabolic processes. In the diet, iron is present in a number of different forms, generally described as haem (from haemoglobin and myoglobin in animal tissue) and non-haem iron (including ferric oxides and salts, ferritin and lactoferrin). This review describes the molecular mechanisms that co-ordinate the absorption of iron from the diet and its release into the circulation. While many components of the iron transport pathway have been elucidated, a number of key issues still remain to be resolved. Future work in this area will provide a clearer picture regarding the transcellular flux of iron and its regulation by dietary and humoral factors.
Figures
References
-
- Hallberg L, Bjørn-Rasmussen E, Howard L, Rossander L. Dietary heme iron absorption. A discussion of possible mechanisms for the absorption-promoting effect of meat and for the regulation of iron absorption. Scand J Gastroenterol. 1979;14:769–779. - PubMed
-
- Hallberg L, Brune M, Rossander L. Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am J Clin Nutr. 1989;49:140–144. - PubMed
-
- Han O, Failla ML, Hill AD, Morris ER, Smith JC. Reduction of Fe(III) is required for uptake of nonheme iron by Caco-2 cells. J Nutr. 1995;125:1291–1299. - PubMed
-
- Glahn RP, Van Campen DR. Iron uptake is enhanced in Caco-2 cell monolayers by cysteine and reduced cysteinyl glycine. J Nutr. 1997;127:642–647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

