Redox-dependent change of nucleotide affinity to the active site of the mammalian complex I
- PMID: 17760425
- PMCID: PMC2258335
- DOI: 10.1021/bi7009822
Redox-dependent change of nucleotide affinity to the active site of the mammalian complex I
Abstract
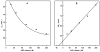
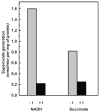
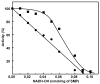
A very potent and specific inhibitor of mitochondrial NADH:ubiquinone oxidoreductase (complex I), a derivative of NADH (NADH-OH) has recently been discovered (Kotlyar, A. B., Karliner, J. S., and Cecchini, G. (2005) FEBS Lett. 579, 4861-4866). Here we present a quantitative analysis of the interaction of NADH-OH and other nucleotides with oxidized and reduced complex I in tightly coupled submitochondrial particles. Both the rate of the NADH-OH binding and its affinity to complex I are strongly decreased in the presence of succinate. The effect of succinate is completely reversed by rotenone, antimycin A, and uncoupler. The relative affinity of ADP-ribose, a competitive inhibitor of NADH oxidation, is also shown to be significantly affected by enzyme reduction (KD of 30 and 500 microM for oxidized and the succinate-reduced enzyme, respectively). Binding of NADH-OH is shown to abolish the succinate-supported superoxide generation by complex I. Gradual inhibition of the rotenone-sensitive uncoupled NADH oxidase and the reverse electron transfer activities by NADH-OH yield the same final titration point (approximately 0.1 nmol/mg of protein). The titration of NADH oxidase appears as a straight line, whereas the titration of the reverse reaction appears as a convex curve. Possible models to explain the different titration patterns for the forward and reverse reactions are briefly discussed.
Figures



Similar articles
-
Generation of superoxide by the mitochondrial Complex I.Biochim Biophys Acta. 2006 May-Jun;1757(5-6):553-61. doi: 10.1016/j.bbabio.2006.03.013. Epub 2006 Apr 17. Biochim Biophys Acta. 2006. PMID: 16678117 Review.
-
A competitive inhibition of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) by ADP-ribose.Biochim Biophys Acta. 1997 Jul 4;1320(3):256-64. doi: 10.1016/s0005-2728(97)00029-7. Biochim Biophys Acta. 1997. PMID: 9230920
-
Pro- and anti-oxidant activities of the mitochondrial respiratory chain: factors influencing NAD(P)H-induced lipid peroxidation.Biochim Biophys Acta. 1997 Jan 16;1318(1-2):246-54. doi: 10.1016/s0005-2728(96)00142-9. Biochim Biophys Acta. 1997. PMID: 9030267
-
Reverse electron transport effects on NADH formation and metmyoglobin reduction.Meat Sci. 2015 Jul;105:89-92. doi: 10.1016/j.meatsci.2015.02.012. Epub 2015 Mar 3. Meat Sci. 2015. PMID: 25828162
-
NADH/NAD+ interaction with NADH: ubiquinone oxidoreductase (complex I).Biochim Biophys Acta. 2008 Jul-Aug;1777(7-8):729-34. doi: 10.1016/j.bbabio.2008.04.014. Epub 2008 Apr 18. Biochim Biophys Acta. 2008. PMID: 18471432 Free PMC article. Review.
Cited by
-
Evaluation of anti-bacterial and wound healing activity of the fruits of Amorpha fruticosa L.Afr J Tradit Complement Altern Med. 2013 Apr 12;10(3):458-68. doi: 10.4314/ajtcam.v10i3.12. eCollection 2013. Afr J Tradit Complement Altern Med. 2013. PMID: 24146475 Free PMC article.
-
Structural Basis for Inhibition of ROS-Producing Respiratory Complex I by NADH-OH.Angew Chem Int Ed Engl. 2021 Dec 20;60(52):27277-27281. doi: 10.1002/anie.202112165. Epub 2021 Nov 15. Angew Chem Int Ed Engl. 2021. PMID: 34612584 Free PMC article.
-
Reduction of the off-pathway iron-sulphur cluster N1a of Escherichia coli respiratory complex I restrains NAD+ dissociation.Sci Rep. 2017 Aug 18;7(1):8754. doi: 10.1038/s41598-017-09345-4. Sci Rep. 2017. PMID: 28821859 Free PMC article.
-
What are the sources of hydrogen peroxide production by heart mitochondria?Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):939-44. doi: 10.1016/j.bbabio.2010.02.013. Epub 2010 Feb 17. Biochim Biophys Acta. 2010. PMID: 20170624 Free PMC article.
-
Molecular identification of the enzyme responsible for the mitochondrial NADH-supported ammonium-dependent hydrogen peroxide production.FEBS Lett. 2011 Jan 21;585(2):385-9. doi: 10.1016/j.febslet.2010.12.019. Epub 2010 Dec 17. FEBS Lett. 2011. PMID: 21168410 Free PMC article.
References
-
- Friedrich T, Steinmuller K, Weiss H. The proton-pumping respiratory complex I of bacteria and mitochondria and its homologue in chloroplasts. FEBS Lett. 1995;367:107–111. - PubMed
-
- Dupuis A, Chevallet M, Darrouzet E, Duborjal H, Lunardi J, Issartel JP. The Complex I from Rhodobacter capsulatus. Biochim Biophys Acta. 1998;1364:147–165. - PubMed
-
- Yagi T, Yano T, Matsuno-Yagi A. Characteristics of the energy-transducing NADH-quinone oxidoreductase of Paracoccus denitrificans as revealed by biochemical, biophysical, and molecular biological approaches. J Bioenerg Biomembr. 1993;25:339–345. - PubMed
-
- Caroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J, Walker JE. Bovine complex I is a complex of 45 different subunits. J Biol Chem. 2006;281:32724–32727. - PubMed
-
- Ragan CI. NADH-ubiquinone oxidoreductase. Biochim Biophys Acta. 1976;456:249–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

