Reducing amyloid plaque burden via ex vivo gene delivery of an Abeta-degrading protease: a novel therapeutic approach to Alzheimer disease
- PMID: 17760499
- PMCID: PMC1952204
- DOI: 10.1371/journal.pmed.0040262
Reducing amyloid plaque burden via ex vivo gene delivery of an Abeta-degrading protease: a novel therapeutic approach to Alzheimer disease
Abstract
Background: Understanding the mechanisms of amyloid-beta protein (Abeta) production and clearance in the brain has been essential to elucidating the etiology of Alzheimer disease (AD). Chronically decreasing brain Abeta levels is an emerging therapeutic approach for AD, but no such disease-modifying agents have achieved clinical validation. Certain proteases are responsible for the catabolism of brain Abeta in vivo, and some experimental evidence suggests they could be used as therapeutic tools to reduce Abeta levels in AD. The objective of this study was to determine if enhancing the clearance of Abeta in the brain by ex vivo gene delivery of an Abeta-degrading protease can reduce amyloid plaque burden.
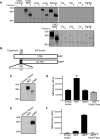
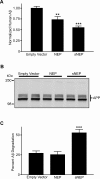
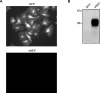
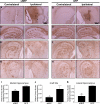
Methods and findings: We generated a secreted form of the Abeta-degrading protease neprilysin, which significantly lowers the levels of naturally secreted Abeta in cell culture. We then used an ex vivo gene delivery approach utilizing primary fibroblasts to introduce this soluble protease into the brains of beta-amyloid precursor protein (APP) transgenic mice with advanced plaque deposition. Brain examination after cell implantation revealed robust clearance of plaques at the site of engraftment (72% reduction, p = 0.0269), as well as significant reductions in plaque burden in both the medial and lateral hippocampus distal to the implantation site (34% reduction, p = 0.0020; and 55% reduction, p = 0.0081, respectively).
Conclusions: Ex vivo gene delivery of an Abeta-degrading protease reduces amyloid plaque burden in transgenic mice expressing human APP. These results support the use of Abeta-degrading proteases as a means to therapeutically lower Abeta levels and encourage further exploration of ex vivo gene delivery for the treatment of Alzheimer disease.
Conflict of interest statement
Figures


Similar articles
-
Neprilysin regulates amyloid Beta peptide levels.J Mol Neurosci. 2004;22(1-2):5-11. doi: 10.1385/JMN:22:1-2:5. J Mol Neurosci. 2004. PMID: 14742905
-
Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice.J Neurosci. 2009 Feb 18;29(7):1977-86. doi: 10.1523/JNEUROSCI.2984-08.2009. J Neurosci. 2009. PMID: 19228952 Free PMC article.
-
Herpes simplex virus RNAi and neprilysin gene transfer vectors reduce accumulation of Alzheimer's disease-related amyloid-beta peptide in vivo.Gene Ther. 2006 Jul;13(14):1068-79. doi: 10.1038/sj.gt.3302719. Epub 2006 Mar 16. Gene Ther. 2006. PMID: 16541122
-
Aβ-degrading enzymes: potential for treatment of Alzheimer disease.J Neuropathol Exp Neurol. 2011 Nov;70(11):944-59. doi: 10.1097/NEN.0b013e3182345e46. J Neuropathol Exp Neurol. 2011. PMID: 22002425 Review.
-
[Gene therapy for Alzheimer' s disease].Nihon Rinsho. 2005 Mar;63(3):394-400. Nihon Rinsho. 2005. PMID: 15773336 Review. Japanese.
Cited by
-
Neprilysin protects against cerebral amyloid angiopathy and Aβ-induced degeneration of cerebrovascular smooth muscle cells.Brain Pathol. 2011 Sep;21(5):594-605. doi: 10.1111/j.1750-3639.2011.00486.x. Epub 2011 Apr 3. Brain Pathol. 2011. PMID: 21382117 Free PMC article.
-
Overexpression of neprilysin reduces alzheimer amyloid-beta42 (Abeta42)-induced neuron loss and intraneuronal Abeta42 deposits but causes a reduction in cAMP-responsive element-binding protein-mediated transcription, age-dependent axon pathology, and premature death in Drosophila.J Biol Chem. 2008 Jul 4;283(27):19066-76. doi: 10.1074/jbc.M710509200. Epub 2008 May 7. J Biol Chem. 2008. PMID: 18463098 Free PMC article.
-
Aggregation and catabolism of disease-associated intra-Abeta mutations: reduced proteolysis of AbetaA21G by neprilysin.Neurobiol Dis. 2008 Sep;31(3):442-50. doi: 10.1016/j.nbd.2008.06.001. Epub 2008 Jun 17. Neurobiol Dis. 2008. PMID: 18602473 Free PMC article.
-
Peripherally expressed neprilysin reduces brain amyloid burden: a novel approach for treating Alzheimer's disease.J Neurosci Res. 2009 May 1;87(6):1462-73. doi: 10.1002/jnr.21944. J Neurosci Res. 2009. PMID: 19021293 Free PMC article.
-
Testing the therapeutic potential of doxycycline in a Drosophila melanogaster model of Alzheimer disease.J Biol Chem. 2011 Dec 2;286(48):41647-41655. doi: 10.1074/jbc.M111.274548. Epub 2011 Oct 13. J Biol Chem. 2011. PMID: 21998304 Free PMC article.
References
-
- Selkoe DJ. Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. - PubMed
-
- D'Amore JD, Kajdasz ST, McLellan ME, Bacskai BJ, Stern EA, et al. In vivo multiphoton imaging of a transgenic mouse model of Alzheimer disease reveals marked thioflavine-S-associated alterations in neurite trajectories. J Neuropathol Exp Neurol. 2003;62:137–145. - PubMed
-
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. - PubMed
-
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

