Molecular targeting of proteins by L-homocysteine: mechanistic implications for vascular disease
- PMID: 17760510
- PMCID: PMC2855132
- DOI: 10.1089/ars.2007.1809
Molecular targeting of proteins by L-homocysteine: mechanistic implications for vascular disease
Abstract
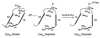
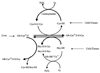
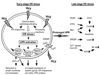
Hyperhomocysteinemia is an independent risk factor for cardiovascular disease, complications of pregnancy, cognitive impairment, and osteoporosis. That elevated homocysteine leads to vascular dysfunction may be the linking factor between these apparently unrelated pathologies. Although a growing body of evidence suggests that homocysteine plays a causal role in atherogenesis, specific mechanisms to explain the underlying pathogenesis have remained elusive. This review focuses on chemistry unique to the homocysteine molecule to explain its inherent cytotoxicity. Thus, the high pKa of the sulfhydryl group (pKa, 10.0) of homocysteine underlies its ability to form stable disulfide bonds with protein cysteine residues, and in the process, alters or impairs the function of the protein. Studies in this laboratory have identified albumin, fibronectin, transthyretin, and metallothionein as targets for homocysteinylation. In the case of albumin, the mechanism of targeting has been elucidated. Homocysteinylation of the cysteine residues of fibronectin impairs its ability to bind to fibrin. Homocysteinylation of the cysteine residues of metallothionein disrupts zinc binding by the protein and abrogates inherent superoxide dismutase activity. Thus, S-homocysteinylation of protein cysteine residues may explain mechanistically the cytotoxicity of elevated L-homocysteine.
Figures






Similar articles
-
Molecular targeting by homocysteine: a mechanism for vascular pathogenesis.Clin Chem Lab Med. 2005;43(10):1076-83. doi: 10.1515/CCLM.2005.188. Clin Chem Lab Med. 2005. PMID: 16197301 Review.
-
The molecular basis of homocysteine thiolactone-mediated vascular disease.Clin Chem Lab Med. 2007;45(12):1704-16. doi: 10.1515/CCLM.2007.338. Clin Chem Lab Med. 2007. PMID: 17937605 Review.
-
Hydrolysis of homocysteine thiolactone results in the formation of Protein-Cys-S-S-homocysteinylation.Proteins. 2019 Aug;87(8):625-634. doi: 10.1002/prot.25681. Epub 2019 Apr 15. Proteins. 2019. PMID: 30869815
-
S-linked protein homocysteinylation: identifying targets based on structural, physicochemical and protein-protein interactions of homocysteinylated proteins.Amino Acids. 2013 May;44(5):1307-16. doi: 10.1007/s00726-013-1465-5. Epub 2013 Feb 12. Amino Acids. 2013. PMID: 23400378
-
Plasma protein homocysteinylation in uremia.Clin Chem Lab Med. 2007;45(12):1678-82. doi: 10.1515/CCLM.2007.336. Clin Chem Lab Med. 2007. PMID: 17937608 Review.
Cited by
-
Role of homocysteinylation of ACE in endothelial dysfunction of arteries.Am J Physiol Heart Circ Physiol. 2015 Jan 15;308(2):H92-100. doi: 10.1152/ajpheart.00577.2014. Epub 2014 Nov 21. Am J Physiol Heart Circ Physiol. 2015. PMID: 25416191 Free PMC article.
-
Tailoring protein nanomechanics with chemical reactivity.Nat Commun. 2017 Jun 6;8:15658. doi: 10.1038/ncomms15658. Nat Commun. 2017. PMID: 28585528 Free PMC article.
-
Chemical and functional aspects of posttranslational modification of proteins.Acta Naturae. 2009 Oct;1(3):29-51. Acta Naturae. 2009. PMID: 22649613 Free PMC article.
-
Development of novel LOXL1 genotyping method and evaluation of LOXL1, APOE and MTHFR polymorphisms in exfoliation syndrome/glaucoma in a Greek population.Mol Vis. 2013 May 6;19:1006-16. Print 2013. Mol Vis. 2013. PMID: 23687437 Free PMC article.
-
Peroxiredoxin post-translational modifications by redox messengers.Redox Biol. 2014 Jun 5;2:777-85. doi: 10.1016/j.redox.2014.06.001. eCollection 2014. Redox Biol. 2014. PMID: 25009779 Free PMC article.
References
-
- Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11 suppl 1:S56–S64. - PubMed
-
- Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochem Int. 1997;31:477–510. discussion 517–476. - PubMed
-
- Benson MD. Amyloidosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill Medical Publishing Division; 2001. pp. 5345–5378.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

