Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides
- PMID: 17760832
- PMCID: PMC3823249
- DOI: 10.1111/j.1582-4934.2007.00062.x
Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides
Abstract
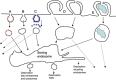
Macropinocytosis defines a series of events initiated by extensive plasma membrane reorganization or ruffling to form an external macropinocytic structure that is then enclosed and internalized. The process is constitutive in some organisms and cell types but in others it is only pronounced after growth factor stimulation. Internalized macropinosomes share many features with phagosomes and both are distinguished from other forms of pinocytic vesicles by their large size, morphological heterogeneity and lack of coat structures. A paucity of information is available on other distinguishing features for macropinocytosis such as specific marker proteins and drugs that interfere with its mechanism over other endocytic processes. This has hampered efforts to characterize the dynamics of this pathway and to identify regulatory proteins that are expressed in order to allow it to proceed. Upon internalization, macropinosomes acquire regulatory proteins common to other endocytic pathways, suggesting that their identities as unique structures are short-lived. There is however less consensus regarding the overall fate of the macropinosome cargo or its limiting membrane and processes such as fusion, tubulation, recycling and regulated exocytosis have all been implicated in shaping the macropinosome and directing cargo traffic. Macropinocytosis has also been implicated in the internalization of cell penetrating peptides that are of significant interest to researchers aiming to utilize their translocation abilities to deliver therapeutic entities such as genes and proteins into cells. This review focuses on recent findings on the regulation of macropinocytosis, the intracellular fate of the macropinosome and discusses evidence for the role of this pathway as a mechanism of entry for cell penetrating peptides.
Figures


References
-
- Marsh M. Endocytosis. Oxford: Oxford University Press; 2001.
-
- Cole NB, Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol. 1995;7:55–64. - PubMed
-
- Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. - PubMed
-
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
-
- Johannes L, Lamaze C. Clathrin-dependent or not: is it still the question? Traffic. 2002;3:443–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

