Close relation of arterial ICC-like cells to the contractile phenotype of vascular smooth muscle cell
- PMID: 17760838
- PMCID: PMC2121184
- DOI: 10.1111/j.1582-4934.2007.00066.x
Close relation of arterial ICC-like cells to the contractile phenotype of vascular smooth muscle cell
Abstract
This work aimed to establish the lineage of cells similar to the interstitial cells of Cajal (ICC), the arterial ICC-like (AIL) cells, which have recently been described in resistance arteries, and to study their location in the artery wall. Segments of guinea-pig mesenteric arteries and single AIL cells freshly isolated from them were used. Confocal imaging of immunostained cells or segments and electron microscopy of artery segments were used to test for the presence and cellular localization of selected markers, and to localize AIL cells in intact artery segments. AIL cells were negative for PGP9.5, a neural marker, and for von Willebrand factor (vWF), an endothelial cell marker. They were positive for smooth muscle alpha-actin and smooth muscle myosin heavy chain (SM-MHC), but expressed only a small amount of smoothelin, a marker of contractile smooth muscle cells (SMC), and of myosin light chain kinase (MLCK), a critical enzyme in the regulation of smooth muscle contraction. Cell isolation in the presence of latrunculin B, an actin polymerization inhibitor, did not cause the disappearance of AIL cells from cell suspension. The fluorescence of basal lamina protein collagen IV was comparable between the AIL cells and the vascular SMCs and the fluorescence of laminin was higher in AIL cells compared to vascular SMCs. Moreover, cells with thin processes were found in the tunica media of small resistance arteries using transmission electron microscopy. The results suggest that AIL cells are immature or phenotypically modulated vascular SMCs constitutively present in resistance arteries.
Figures

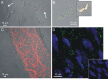
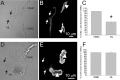


References
-
- Campbell GR, Campbell JH, Manderson JA, Horrigan S, Rennick RE. Arterial smooth muscle: a multifunctional mesenchymal cell. Arch Pathol Lab Med. 1988;112:977–86. - PubMed
-
- Chamley-Campbell JH, Campbell GR. Wha? controls smooth muscle phenotype? Atherosclerosis. 1981;40:347–57. - PubMed
-
- Shanahan CM, Weissberg PL. Smooth muscle cell heterogeneity. Patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1998;18:333–8. - PubMed
-
- Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling. From innocent bystander to active participant. Circ Res. 2001;89:1111–21. - PubMed
-
- Halayko AJ, Solway J. Plasticity in skeletal, cardiac, and smooth muscle. Invited review: molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

