Studies on brain volume, Alzheimer-related proteins and cytokines in mice with chronic overexpression of IL-1 receptor antagonist
- PMID: 17760842
- PMCID: PMC3823259
- DOI: 10.1111/j.1582-4934.2007.00074.x
Studies on brain volume, Alzheimer-related proteins and cytokines in mice with chronic overexpression of IL-1 receptor antagonist
Abstract
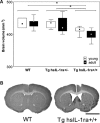
Inflammation is associated with both acute and chronic neurological disorders, including stroke and Alzheimer's disease (AD). Cytokines such as interleukin (IL)-1 have several activities in the brain both under physiological and pathophysiological conditions. The objective of this study was to evaluate consequences of the central blockade of IL-1 transmission in a previously developed transgenic mouse strain with brain-directed overexpression of human soluble IL-1 receptor antagonist (Tg hsIL-1ra). Effects on brain morphology and brain levels of the AD-related proteins beta-amyloid precursor protein (APP) and presenilin 1(PS1), as well as the levels of IL-1beta, IL-6 and tumour necrosis factor-alpha (TNF-alpha) were analysed in homozygotic and heterozygotic mice and wild type (WT) controls, of both genders and of young (30-40 days) and adult (13-14 months) age. A marked reduction in brain volume was observed in transgenic mice as determined by volumetry. Western blot analysis showed higher levels of APP, but lower levels of PS1, in adult animals than in young ones. In the cerebellum, heterozygotic (Tg hsIL-1ra(+/-)) mice had lower levels of APP and PS1 than WT mice. With one exception, there were no genotypic differences in the levels of IL-1beta, IL-6 and TNF-alpha. The cytokine levels were generally higher in adult than in young mice. In conclusion, the chronic blockade of IL-1 signalling in the brain was associated with an atrophic phenotype of the brain, and with modified levels of APP and PS1. Brain-directed overexpression of hsIL-1ra was not followed by major compensatory changes in the levels of pro-inflammatory cytokines.
Figures





References
-
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–25. - PubMed
-
- Minami M, Kuraishi Y, Yabuuchi K, Yamazaki A, Satoh M. Induction of interleukin-1β mRNA in rat brain after transient forebrain ischemia. J Neurochem. 1992;58:390–2. - PubMed
-
- Stroemer RP, Rothwell NJ. Exacerbation of ischemic brain damage by localized striatal injection of interleukin- 1β in the rat. J Cereb Blood Flow Metab. 1998;18:833–9. - PubMed
-
- Blume AJ, Vitek MP. Focusing on IL-1-promotion of β-amyloid precursor protein synthesis as an early event in Alzheimer's disease. Neurobiol Aging. 1989;10:406–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

