Transient down-regulation of beta1 integrin subtypes on kidney carcinoma cells is induced by mechanical contact with endothelial cell membranes
- PMID: 17760843
- PMCID: PMC3823260
- DOI: 10.1111/j.1582-4934.2007.00071.x
Transient down-regulation of beta1 integrin subtypes on kidney carcinoma cells is induced by mechanical contact with endothelial cell membranes
Abstract
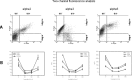
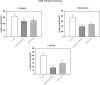
Adhesion molecules of the integrin beta1 family are thought to be involved in the malignant progression renal cell carcinoma (RCC). Still, it is not clear how they contribute to this process. Since the hematogenous phase of tumour dissemination is the rate-limiting step in the metastatic process, we explored beta1 integrin alterations on several RCC cell lines (A498, Caki1, KTC26) before and after contacting vascular endothelium in a tumour-endothelium (HUVEC) co-culture assay. Notably, alpha2, alpha3 and alpha5 integrins became down-regulated immediately after the tumour cells attached to HUVEC, followed by re-expression shortly thereafter. Integrin down-regulation on RCC cells was caused by direct contact with endothelial cells, since the isolated endothelial membrane fragments but not the cell culture supernatant contributed to the observed effects. Integrin loss was accompanied by a reduced focal adhesion kinase (FAK) expression, FAK activity and diminished binding of tumour cells to matrix proteins. Furthermore, intracellular signalling proteins RCC cells were altered in the presence of HUVEC membrane fragments, in particular 14-3-3 epsilon, ERK2, PKCdelta, PKCepsilon and RACK1, which are involved in regulating tumour cell motility. We, therefore, speculate that contact of RCC cells with the vascular endothelium converts integrin-dependent adhesion to integrin-independent cell movement. The process of dynamic integrin regulation may be an important part in tumour cell migration strategy, switching the cells from being adhesive to becoming motile and invasive.
Figures






Similar articles
-
Migration of renal carcinoma cells is dependent on protein kinase Cdelta via beta1 integrin and focal adhesion kinase.Int J Oncol. 2008 May;32(5):1125-31. Int J Oncol. 2008. PMID: 18425341
-
Altered expression of beta1 integrins in renal carcinoma cell lines exposed to the differentiation inducer valproic acid.Int J Mol Med. 2006 Aug;18(2):347-54. Int J Mol Med. 2006. PMID: 16820945
-
CXCR4 chemokine receptor engagement modifies integrin dependent adhesion of renal carcinoma cells.Exp Cell Res. 2007 Nov 15;313(19):4051-65. doi: 10.1016/j.yexcr.2007.07.001. Epub 2007 Jul 10. Exp Cell Res. 2007. PMID: 17706641
-
Regulation of beta1 integrin expression by PKCepsilon in renal cancer cells.Int J Oncol. 2004 Oct;25(4):1157-63. Int J Oncol. 2004. PMID: 15375568
-
The integrin adhesome and control of anti-tumour immunity.Biochem Soc Trans. 2024 Dec 19;52(6):2455-2468. doi: 10.1042/BST20240386. Biochem Soc Trans. 2024. PMID: 39641590 Free PMC article. Review.
Cited by
-
Glioma-associated endothelial cells are chemoresistant to temozolomide.J Neurooncol. 2009 Oct;95(1):13-22. doi: 10.1007/s11060-009-9891-7. Epub 2009 Apr 18. J Neurooncol. 2009. PMID: 19381445
-
[Preclinical studies on the influence of the tyrosine kinase inhibitor AEE788 on malignant properties of renal cell carcinoma cells].Urologe A. 2008 Sep;47(9):1175-81. doi: 10.1007/s00120-008-1831-1. Urologe A. 2008. PMID: 18688594 German.
-
Valproic acid blocks adhesion of renal cell carcinoma cells to endothelium and extracellular matrix.J Cell Mol Med. 2009 Aug;13(8B):2342-2352. doi: 10.1111/j.1582-4934.2008.00603.x. J Cell Mol Med. 2009. PMID: 19067765 Free PMC article.
-
EphA2 Is a Potential Player of Malignant Cellular Behavior in Non-Metastatic Renal Cell Carcinoma Cells but Not in Metastatic Renal Cell Carcinoma Cells.PLoS One. 2015 Jul 15;10(7):e0130975. doi: 10.1371/journal.pone.0130975. eCollection 2015. PLoS One. 2015. PMID: 26177500 Free PMC article.
-
Alterations of the gene expression profile in renal cell carcinoma after treatment with the histone deacetylase-inhibitor valproic acid and interferon-alpha.World J Urol. 2011 Dec;29(6):779-86. doi: 10.1007/s00345-010-0582-y. Epub 2010 Jul 17. World J Urol. 2011. PMID: 20640575
References
-
- Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–83. - PubMed
-
- Tawil NJ, Gowri V, Djoneidi M, Nip J, Carbonetto S, Brodt P. Integrin alpha3beta1 can promote adhesion and spreading of metastatic breast carcinoma cells on the lymph node stroma. Int J Cancer. 1996;66:703–10. - PubMed
-
- Seftor RE, Seftor EA, Sheng S, Pemberton PA, Sager R, Hendrix MJ. Maspin suppresses the invasive phenotype of human breast carcinoma. Cancer Res. 1998;58:5681–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

